Selinexor is an oral small molecule Selective Inhibitor of Nuclear Export (SINE) that blocks the transport protein exportin-1 (XPO1). By inhibiting XPO1, selinexor prevents the nuclear export of viral ribonucleoproteins and restores the nuclear localization of host anti-inflammatory and tumor suppressor proteins, theoretically reducing viral replication and mitigating cytokine storm.
Recent:Geils.
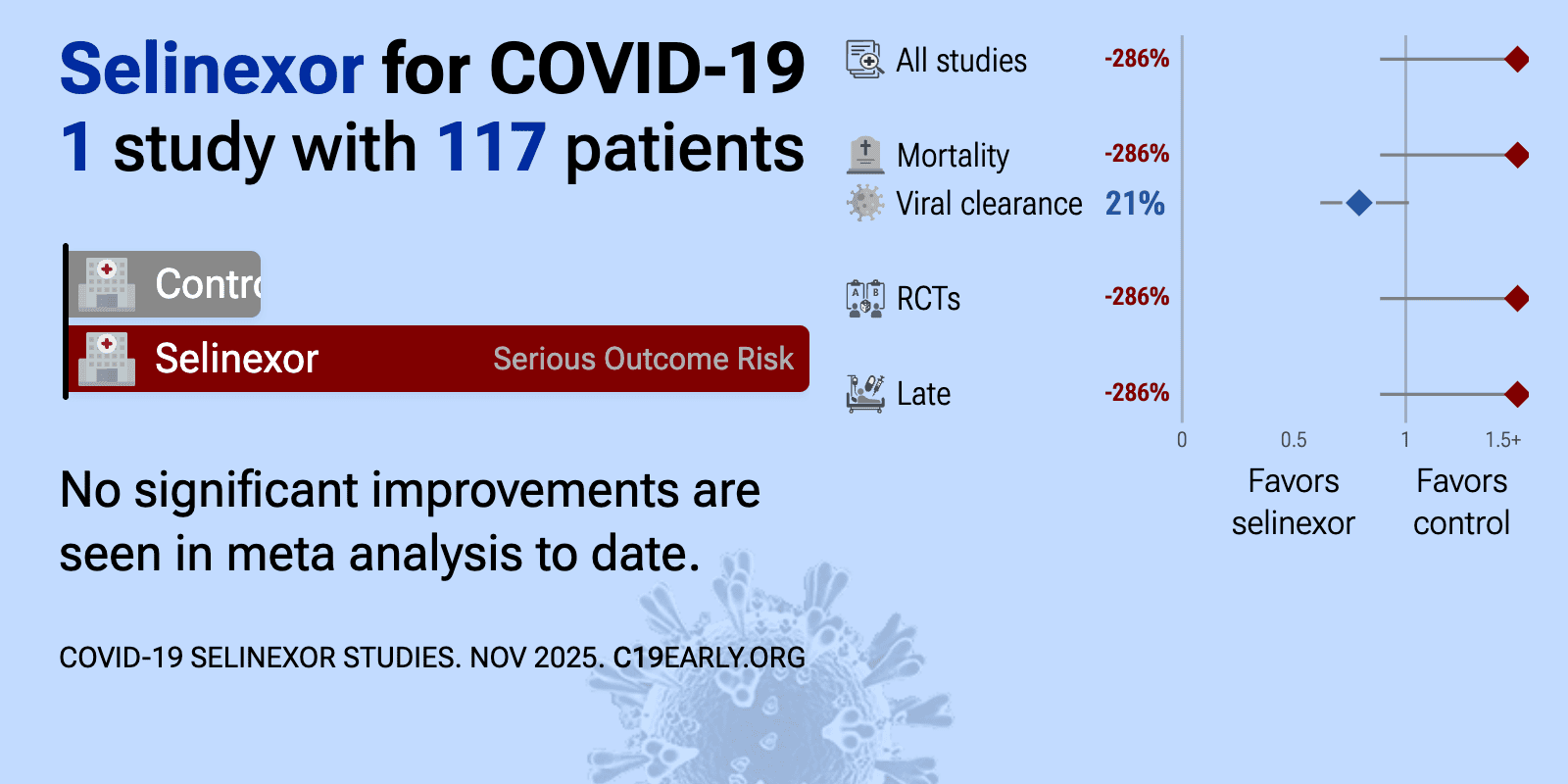
Oct 10 2020 |
et al., Karyopharm Therapeutics | Treatment of Severe COVID-19 with Low-Dose Selinexor: Demonstration of Anti-Viral and Anti-Inflammatory Activities in a Randomized, International, Multicenter, Placebo-Controlled Phase 2 Clinical Trial |
| 286% higher mortality (p=0.07), 39% higher ventilation (p=0.58), 11% higher ICU admission (p=0.71), and 16% lower hospital discharge (p=0.68). RCT 188 hospitalized patients with severe COVID-19 showing higher mortality (p=0.07) and no significant clinical improvement with oral selinexor (20mg) compared to placebo. In the intention-to-treat analysis, the study failed its primary .. | ||