Vitamin D, D-binding protein, free vitamin D and COVID-19 mortality in hospitalized patients
et al., The American Journal of Clinical Nutrition, doi:10.1093/ajcn/nqac027, Jan 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 427 hospitalized COVID-19 patients in the United Kingdom, showing lower mortality with vitamin D supplementation (p=0.12), and higher mortality with both low and high vitamin D levels compared to a reference range of 50-74 nmol/L.
This is the 68th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
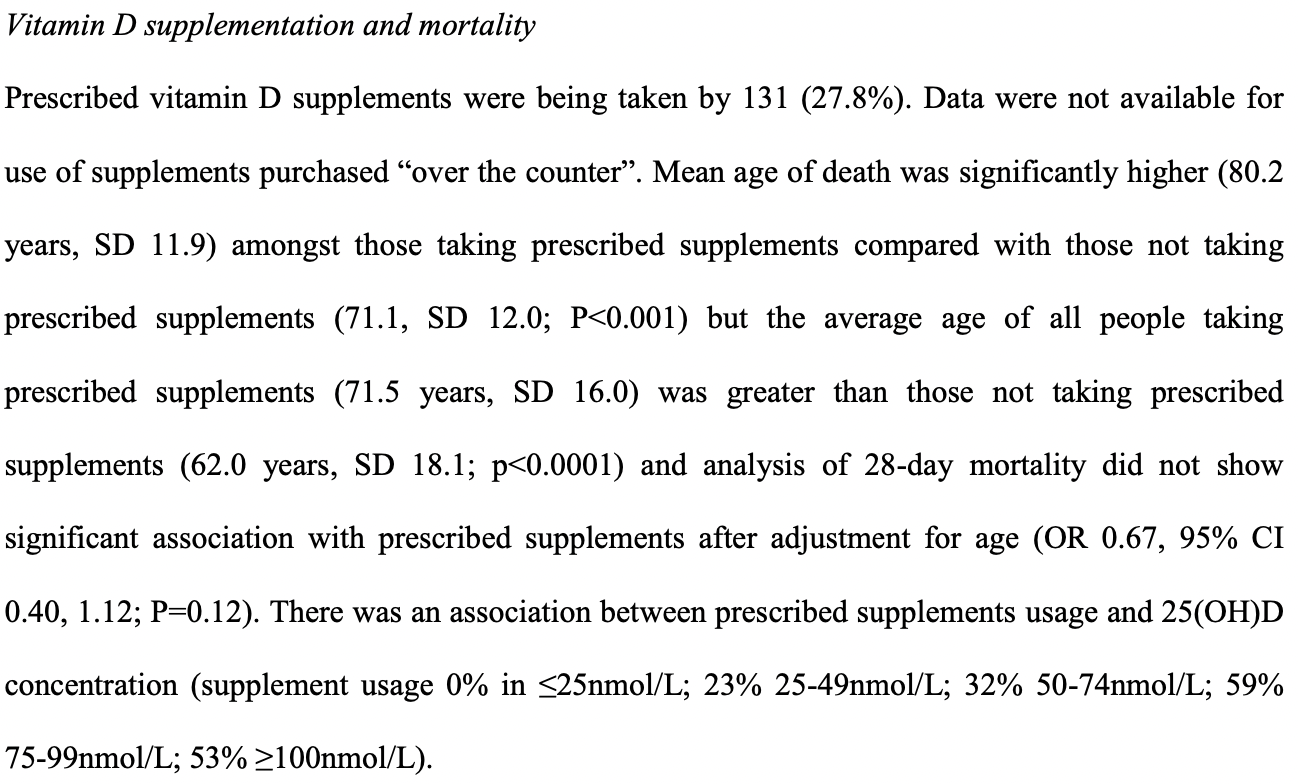
risk of death, 27.3% lower, RR 0.73, p = 0.12, treatment 31 of 131 (23.7%), control 80 of 336 (23.8%), adjusted per study, odds ratio converted to relative risk, prescribed supplement use, multivariable.
|
|
risk of death, 49.7% lower, RR 0.50, p = 0.02, high D levels 16 of 115 (13.9%), low D levels 33 of 118 (28.0%), NNT 7.1, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, 50-74 nmol/L vs. <25nmol/L, multivariable, outcome based on serum levels.
|
|
risk of death, 39.7% lower, RR 0.60, p = 0.07, high D levels 16 of 115 (13.9%), low D levels 38 of 157 (24.2%), NNT 9.7, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, 50-74 nmol/L vs. 25-49nmol/L, multivariable, outcome based on serum levels.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Subramanian et al., 31 Jan 2022, prospective, United Kingdom, peer-reviewed, 16 authors, dosage not specified.
Abstract: Vitamin D, D-binding protein, free vitamin D and COVID-19
mortality in hospitalized patients.
Short title: Vitamin D and COVID-19 mortality
PT
Martin Hewison5, Rene F Chun6, Andrea Jorgensen7, Paul Richardson1, Darshan Nitchingham1,
RI
Joseph Aslan1, Maya Shah1, Coonoor R Chandrasekar8, Amanda Wood9, Mike Beadsworth10,
Department of Gastroenterology, Liverpool University Hospital Foundation NHS Trust, Liverpool,
N
U
1
SC
Munir Pirmohamed11
Department of Cellular and Molecular Physiology, Institute of Translational Medicine, University
M
2
A
UK
3
IT
ED
of Liverpool
Department of Clinical Chemistry, Liverpool University Hospital Foundation NHS Trust,
Liverpool, UK
Department of Biostatistics, Institute of Translational Medicine, University of Liverpool,
ED
4
N
Liverpool, UK
Institute of Metabolism and Systems Research, University of Birmingham
6
Department of Orthopaedic Surgery, University of California, Los Angeles
7
Department of Health Data Science, University of Liverpool, Liverpool, UK
8
Department of Orthopaedic Surgery, Liverpool University Hospital Foundation NHS Trust,
IN
A
L
U
5
Department of Clinical Pharmacology, Liverpool University Hospital Foundation NHS Trust,
O
RI
9
G
Liverpool, UK
Liverpool, UK
© Crown copyright 2022. This article contains public sector information licensed under the Open Government Licence v3.0
(http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/).
Sreedhar Subramanian1, 2 ¶, Jonathan M Rhodes2, , Joseph M Taylor3, Anna M Milan3, Steven Lane4,
10
Tropical and Infectious Diseases Unit, Liverpool University Hospital Foundation NHS Trust,
Liverpool, UK
11
Department of Molecular and Clinical Pharmacology, University of Liverpool
¶
Corresponding author:
Consultant Gastroenterologist and Honorary Senior Lecturer
PT
Department of Gastroenterology
RI
Liverpool University Hospital Foundation NHS Trust and University of Liverpool
SC
Prescot Street
Liverpool L7 8XP, UK
N
U
Email: sreedhar.subramanian@liverpoolft.nhs.uk
A
Acknowledgements: None
M
Registration: The study protocol was approved by the London-Surrey Research Ethics Committee
IT
ED
(20/HRA/2282).
Funding Source: This research was funded by an internal departmental grant from Liverpool
ED
University Hospitals NHS Foundation Trust. The funders had no input into study design or final
Guarantor of the article
A
L
U
Dr Sreedhar Subramanian
N
analysis.
IN
Author contributions: SS, JR, MP, JT and AM were involved in study design and initial drafting
G
of the manuscript. SL was involved in data analysis. All authors revised and approved the final
O
RI
version of the manuscript.
Sreedhar Subramanian, MD, FRCP
Conflict of interest: JMR with the University of Liverpool and Provexis UK, holds a patent for
use of a soluble fibre preparation as maintenance therapy for Crohn’s disease plus a patent for its
use in antibiotic-associated diarrhoea. Patent also held with the University of Liverpool and others
in relation to use of modified heparins in cancer therapy. SS has received speaker fees from MSD,
Actavis, Abbvie, Dr Falk pharmaceuticals, Janssen, Takeda, Boehringer-Ingelheim, Shire and
Abbvie, Dr Falk..
DOI record:
{
"DOI": "10.1093/ajcn/nqac027",
"ISSN": [
"0002-9165",
"1938-3207"
],
"URL": "http://dx.doi.org/10.1093/ajcn/nqac027",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Vitamin D deficiency has been associated with worse coronavirus disease 2019 (COVID-19) outcomes but circulating 25-hydroxyvitamin D (25(OH)D) is largely bound to vitamin D binding protein (DBP) or albumin both of which tend to fall in illness making 25(OH)D status hard to interpret. Because of this, measurement of unbound “free” and albumin-bound “bioavailable” 25(OH) D has been proposed.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>We aimed to examine the relationship between vitamin D status and mortality from COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this observational study conducted in Liverpool, UK, hospitalized COVID-19 patients with surplus sera available for 25(OH)D analysis were studied. Clinical data including age, ethnicity and co-morbidities were extracted from case notes. Serum 25(OH)D, DBP and albumin concentrations were measured. Free and bioavailable 25(OH) D were calculated. Relationships between total, free and bioavailable 25(OH)D and 28-day mortality were analyzed by logistic regression.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Findings</jats:title>\n <jats:p>Four hundred and seventy-two patients with COVID-19 were included, of whom 112 (23.7%) died within 28 days. Non-survivors were older (mean age 73, [range 34-98]) than survivors (65, [19-95]; P = 0.003) and more likely male (67%; P = 0.02). The frequency of vitamin D deficiency (25(OH)D &lt;50nmol/L) was similar between non-survivors (71/112 [63.4%]) and survivors (204/360 [56.7%]; P = 0.15) but, after adjustment for age, sex and co-morbidities, increased odds for mortality were present in those with severe deficiency (25(OH)D &lt;25nmol/L), OR 2.37 (95% CI 1.17, 4.78), or high 25(OH)D (≥100nmol/L), OR 4.65 (95% CI 1.51, 14.34) compared with 25(OH)D 50-74 nmol/L (reference). Serum DBP levels were not associated with mortality after adjustment for 25(OH)D, age, sex and co-morbidities. Neither free nor bioavailable 25(OH)D were associated with mortality.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>: Vitamin D deficiency as commonly defined by serum 25 (OH)D levels (&lt;50nmol/L) is not associated with increased mortality from COVID-19 but extreme low (&lt;25nmol/L) and high (&gt;100nmol/L) levels may be associated with risk. Neither free nor bioavailable 25(OH) D associate with risk.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6483-1730",
"affiliation": [
{
"name": "Department of Gastroenterology, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
},
{
"name": "Department of Cellular and Molecular Physiology, Institute of Translational Medicine, University of Liverpool"
}
],
"authenticated-orcid": false,
"family": "Subramanian",
"given": "Sreedhar",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Cellular and Molecular Physiology, Institute of Translational Medicine, University of Liverpool"
}
],
"family": "Rhodes",
"given": "Jonathan M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Chemistry, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Taylor",
"given": "Joseph M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Chemistry, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Milan",
"given": "Anna M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Institute of Translational Medicine, University of Liverpool, Liverpool, UK"
}
],
"family": "Lane",
"given": "Steven",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5806-9690",
"affiliation": [
{
"name": "Institute of Metabolism and Systems Research, University of Birmingham"
}
],
"authenticated-orcid": false,
"family": "Hewison",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthopaedic Surgery, University of California, Los Angeles"
}
],
"family": "Chun",
"given": "Rene F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health Data Science, University of Liverpool, Liverpool, UK"
}
],
"family": "Jorgensen",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Richardson",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Nitchingham",
"given": "Darshan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Aslan",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Shah",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthopaedic Surgery, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Chandrasekar",
"given": "Coonoor R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacology, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Wood",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tropical and Infectious Diseases Unit, Liverpool University Hospital Foundation NHS Trust, Liverpool, UK"
}
],
"family": "Beadsworth",
"given": "Mike",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7534-7266",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, University of Liverpool"
}
],
"authenticated-orcid": false,
"family": "Pirmohamed",
"given": "Munir",
"sequence": "additional"
}
],
"container-title": [
"The American Journal of Clinical Nutrition"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
27
]
],
"date-time": "2022-01-27T20:11:33Z",
"timestamp": 1643314293000
},
"deposited": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T19:08:49Z",
"timestamp": 1643742529000
},
"funder": [
{
"DOI": "10.13039/100010417",
"doi-asserted-by": "publisher",
"name": "University Hospital Southampton NHS Foundation Trust"
}
],
"indexed": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T19:41:36Z",
"timestamp": 1643744496536
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0002-9165"
},
{
"type": "electronic",
"value": "1938-3207"
}
],
"issued": {
"date-parts": [
[
2022,
1,
31
]
]
},
"language": "en",
"link": [
{
"URL": "https://academic.oup.com/ajcn/advance-article-pdf/doi/10.1093/ajcn/nqac027/42357837/nqac027.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ajcn/advance-article-pdf/doi/10.1093/ajcn/nqac027/42357837/nqac027.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
1,
31
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
31
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Nutrition and Dietetics",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": [
"Vitamin D, D-binding protein, free vitamin D and COVID-19 mortality in hospitalized patients"
],
"type": "journal-article"
}
