Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers
et al., International Journal of Molecular Sciences, doi:10.3390/ijms24087445, Apr 2023
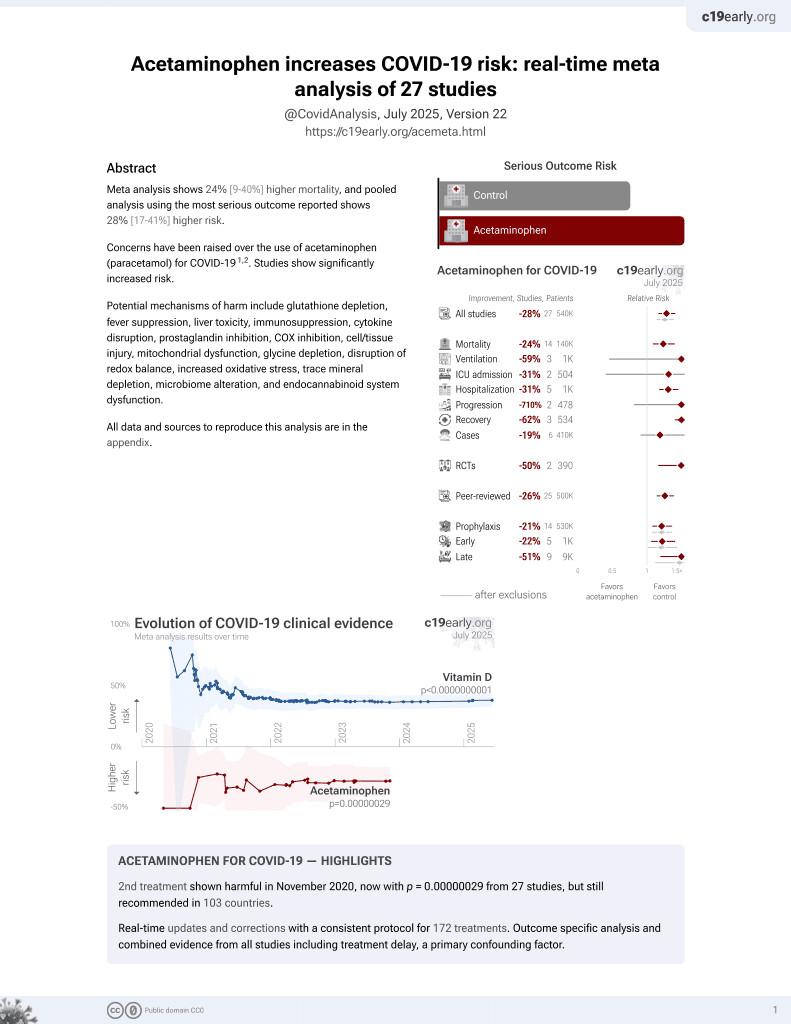
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 80 mild COVID-19 patients in Italy, showing no significant difference in long COVID with acetaminophen use during infection.
Acetaminophen is also known as paracetamol, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
|
risk of long COVID, 18.5% higher, RR 1.19, p = 0.62, treatment 11 of 23 (47.8%), control 23 of 57 (40.4%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Stufano et al., 18 Apr 2023, retrospective, Italy, peer-reviewed, 7 authors.
Contact: annamaria.sardanelli@uniba.it (corresponding author).
Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers
International Journal of Molecular Sciences, doi:10.3390/ijms24087445
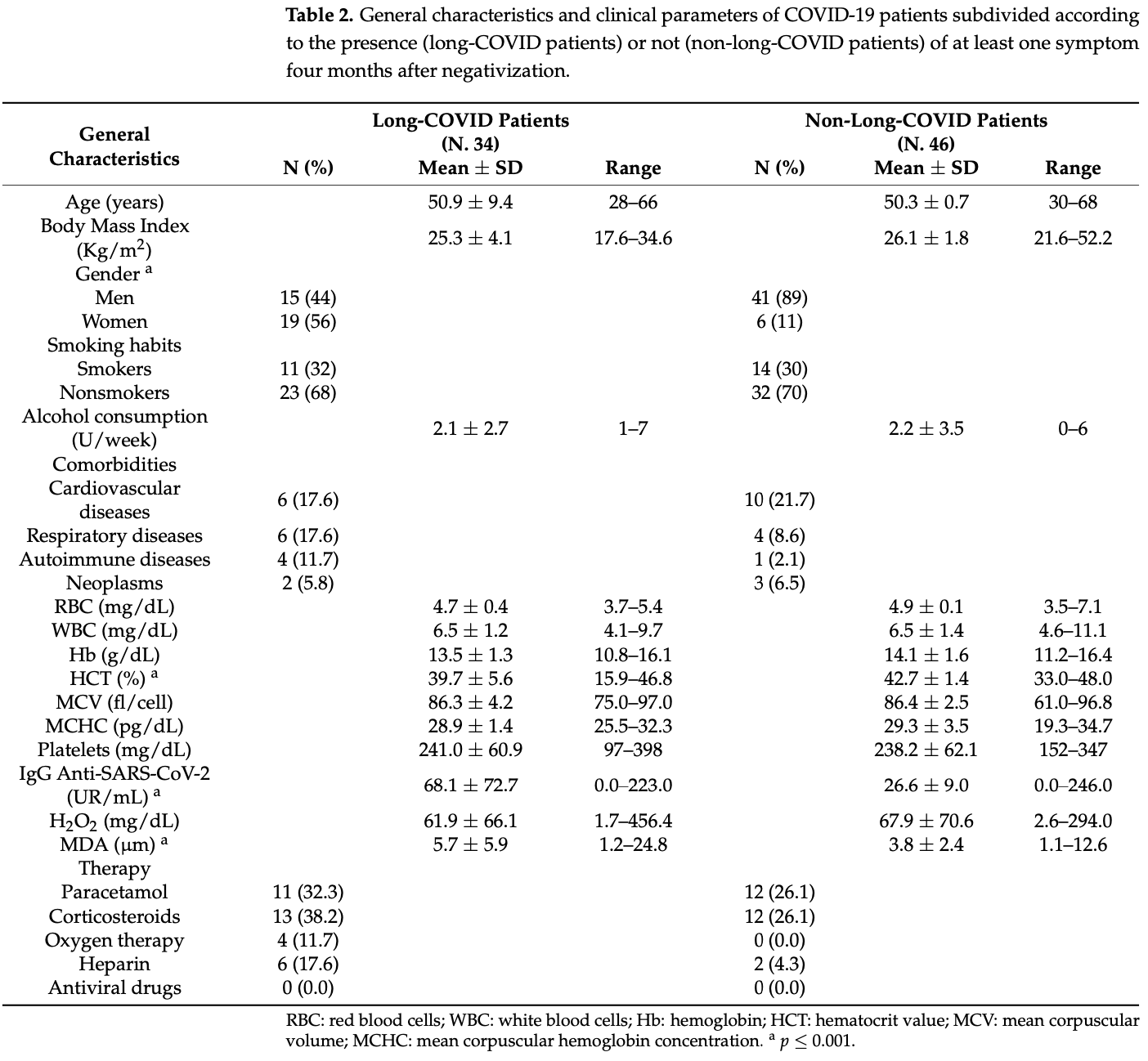
In addition to the acute symptoms after infection, patients and society are also being challenged by the long-term effects of COVID-19, known as long COVID. Oxidative stress, as a pivotal point in the pathophysiology of COVID-19, could potentially be also involved in the development of the post-COVID syndrome. The aim of the present study was to evaluate the relationship between changes in oxidative status and the persistence of long-COVID symptoms in workers with a previous mild COVID-19 infection. A cross-sectional study was conducted among 127 employees of an Italian university (80 with a previous COVID-19 infection, and 47 healthy subjects). The TBARS assay was used to detect malondialdehyde serum levels (MDA), while total hydroperoxide (TH) production was measured by a d-ROMs kit. A significant difference in mean serum MDA values was found between previously infected subjects and healthy controls and (4.9 µm vs. 2.8 µm, respectively). Receiver-operating characteristic (ROC) curves showed high specificity and good sensibility (78.7% and 67.5%, respectively) for MDA serum levels. A random forest classifier identified the hematocrit value, MDA serum levels, and IgG titer against SARS-CoV-2 as features with the highest predictive value in distinguishing 34 long-COVID from 46 asymptomatic post-COVID subjects. Oxidative damage persists in subjects with previous COVID-19 infection, suggesting a possible role of oxidative stress mediators in the pathogenesis of long COVID.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest.
References
Al-Aly, Xie, Bowe, High-dimensional characterization of post-acute sequelae of COVID-19, Nature, doi:10.1038/s41586-021-03553-9
Al-Hakeim, Al-Rubaye, Al-Hadrawi, Almulla, Maes, Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study, Mol. Psychiatry, doi:10.1038/s41380-022-01836-9
Avila-Nava, Pech-Aguilar, Lugo, Medina-Vera, Guevara-Cruz et al., Oxidative Stress Biomarkers and Their Association with Mortality among Patients Infected with SARS-CoV-2 in Mexico, Oxid. Med. Cell. Longev
Ayala, Muñoz, Argüelles, Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal, Oxid. Med. Cell. Longev, doi:10.1155/2014/360438
Biswas, Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox?, Oxid. Med. Cell. Longev, doi:10.1155/2016/5698931
Buonocore, Perrone, Longini, Terzuoli, Bracci, Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies, Pediatr. Res, doi:10.1203/00006450-200002000-00012
Cecchini, Cecchini, SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression, Med. Hypotheses, doi:10.1016/j.mehy.2020.110102
Chang, Chen, Yü, Peng, Peng, Curcumin-Protected PC12 Cells Against Glutamate-Induced Oxidative Toxicity, Food Technol. Biotechnol, doi:10.17113/ftb.52.04.14.3622
Deer, Rock, Vasilevsky, Carmody, Rando et al., Characterizing Long COVID: Deep Phenotype of a Complex Condition, EBioMedicine, doi:10.1016/j.ebiom.2021.103722
Dennis, Wamil, Alberts, Oben, Cuthbertson et al., study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study, BMJ Open, doi:10.1136/bmjopen-2020-048391
Fogh, Larsen, Hansen, Hasselbalch, Eriksen et al., Self-Reported Long COVID and Its Association with the Presence of SARS-CoV-2 Antibodies in a Danish Cohort up to 12 Months after Infection, Microbiol. Spectr, doi:10.1128/spectrum.02537-22
Gadotti, Lipinski, Vasconcellos, Marqueze, Cunha et al., Susceptibility of the patients infected with Sars-Cov2 to oxidative stress and possible interplay with severity of the disease, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2021.01.044
Goldhaber, Kohn, Ogan, Sitapati, Longhurst et al., Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph192416841
Gonzalo, Brieva, Tatzber, Jové, Cacabelos et al., Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism, J. Neurochem, doi:10.1111/j.1471-4159.2012.07934.x
Groff, Sun, Ssentongo, Ba, Parsons et al., Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.28568
Group, Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study, Lancet Respir. Med
Higashi, Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease, Antioxidants, doi:10.3390/antiox11101958
Huang, Huang, Wang, Li, Ren et al., 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study, Lancet, doi:10.1016/S0140-6736(20)32656-8
Isgrò, Spagnuolo, Pannucci, Mondello, Santi et al., Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson's Disease Patients and THP-1 Macrophages, Int. J. Mol. Sci, doi:10.3390/ijms232112774
Ivanov, Valuev-Elliston, Ivanova, Kochetkov, Starodubova et al., Oxidative Stress during HIV Infection: Mechanisms and Consequences, Oxid. Med. Cell. Longev, doi:10.1155/2016/8910396
Jia, Qin, Zhao, Chai, Yu et al., Redox homeostasis maintained by GPX4 facilitates STING activation, Nat. Immunol, doi:10.1038/s41590-020-0699-0
Jové, Mota-Martorell, Pradas, Martín-Gari, Ayala et al., The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity, Antioxidants, doi:10.3390/antiox9111132
Karki, Kanneganti, Innate immunity, cytokine storm, and inflammatory cell death in COVID-19, J. Transl. Med, doi:10.1186/s12967-022-03767-z
Khomich, Kochetkov, Bartosch, Ivanov, Redox Biology of Respiratory Viral Infections, Viruses, doi:10.3390/v10080392
Komaravelli, Casola, Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses, J. Pharm. Pharm
Kosanovic, Sagic, Djukic, Pljesa-Ercegovac, Savic-Radojevic et al., Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings, Antioxidants, doi:10.3390/antiox10071126
Kumar, Thambiraja, Karuppanan, Subramaniam, Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein, J. Med. Virol, doi:10.1002/jmv.27526
Laforge, Elbim, Frère, Hémadi, Massaad et al., Tissue damage from neutrophilinduced oxidative stress in COVID-19, Nat. Rev. Immunol, doi:10.1038/s41577-020-0407-1
Ledur, Karmirian, Pedrosa, Souza, Assis-De-Lemos et al., Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes, Sci. Rep, doi:10.1038/s41598-020-57914-x
Lim, Oh, Kim, Jung, Kim et al., Enhanced oxidative damage to DNA, lipids, and proteins and levels of some antioxidant enzymes, cytokines, and heat shock proteins in patients infected with influenza H1N1 virus, Acta Virol, doi:10.4149/av_2014_03_253
Loconsole, Centrone, Morcavallo, Campanella, Sallustio et al., Rapid Spread of the SARS-CoV-2 Variant of Concern 202012/01 in Southern Italy (December 2020, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph18094766
Lopez-Leon, Wegman-Ostrosky, Perelman, Sepulveda, Rebolledo et al., More than 50 long-term effects of COVID-19: A systematic review and meta-analysis, Sci. Rep, doi:10.1038/s41598-021-95565-8
Lucchese, Vogelgesang, Boesl, Raafat, Holtfreter et al., Anti-neuronal antibodies against brainstem antigens are associated with COVID-19, EBioMedicine, doi:10.1016/j.ebiom.2022.104211
Martín-Fernández, Aller, Heredia-Rodríguez, Gómez-Sánchez, Martínez-Paz et al., Lipid peroxidation as a hallmark of severity in COVID-19 patients, Redox Biol, doi:10.1016/j.redox.2021.102181
Medini, Zirman, Mishmar, Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis, iScience, doi:10.1016/j.isci.2021.103471
Medvedev, Ploen, Hildt, HCV and Oxidative Stress: Implications for HCV Life Cycle and HCV-Associated Pathogenesis, Oxid. Med. Cell. Longev, doi:10.1155/2016/9012580
Mehri, Rahbar, Ghane, Souri, Esfahani, Changes in oxidative markers in COVID-19 patients, Arch. Med. Res, doi:10.1016/j.arcmed.2021.06.004
Merad, Martin, Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages, Nat. Rev. Immunol, doi:10.1038/s41577-020-0331-4
Molnar, Varnai, Schranz, Zavori, Peterfi et al., Severe Fatigue and Memory Impairment Are Associated with Lower Serum Level of Anti-SARS-CoV-2 Antibodies in Patients with Post-COVID Symptoms, J. Clin. Med, doi:10.3390/jcm10194337
Munblit, O'hara, Akrami, Perego, Olliaro et al., Long COVID: Aiming for a consensus, Lancet Respir. Med, doi:10.1016/S2213-2600(22)00135-7
Nalbandian, Sehgal, Gupta, Madhavan, Mcgroder et al., Post-acute COVID-19 syndrome, Nat. Med, doi:10.1038/s41591-021-01283-z
Nersesjan, Amiri, Lebech, Roed, Mens et al., Central and peripheral nervous system complications of COVID-19: A prospective tertiary center cohort with 3-month follow-up, J. Neurol, doi:10.1007/s00415-020-10380-x
Paliogiannis, Fois, Sotgia, Mangoni, Zinellu et al., Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis, Biomark. Med, doi:10.2217/bmm-2017-0420
Palmer, Innate metabolic responses against viral infections, Nat. Metab, doi:10.1038/s42255-022-00652-3
Paterson, Brown, Benjamin, Nortley, Wiethoff et al., The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings, Brain, doi:10.1093/brain/awaa240
Paul, Lemle, Komaroff, Snyder, Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2024358118
Phetsouphanh, Darley, Wilson, Howe, Munier et al., Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection, Nat. Immunol, doi:10.1038/s41590-021-01113-x
Plantone, Locci, Bergantini, Manco, Cortese et al., Brain neuronal and glialdamageduring acute COVID-19 infection in absence of clinical neurologicalmanifestations, J. Neurol. Neurosurg. Psychiatry
Pretorius, Vlok, Venter, Bezuidenhout, Laubscher et al., Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin, Cardiovasc. Diabetol, doi:10.1186/s12933-021-01359-7
Rahal, Kumar, Singh, Yadav, Tiwari et al., Oxidative stress, prooxidants, and antioxidants: The interplay, BioMed Res. Int, doi:10.1155/2014/761264
Stockwell, ; Friedmann, Angeli, Bayir, Bush et al., Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease, Cell, doi:10.1016/j.cell.2017.09.021
Stufano, Lucchese, Stahl, Grattagliano, Dassisti et al., Impact of COVID-19 emergency on the psychological well-being of susceptible individuals, Sci. Rep, doi:10.1038/s41598-022-15357-6
Suhail, Zajac, Fossum, Lowater, Mccracken et al., Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review, Protein J, doi:10.1007/s10930-020-09935-8
Van Eijk, Tami, Hillebrands, Den Dunnen, De Borst et al., Mild Coronavirus Disease 2019 (COVID-19) Is Marked by Systemic Oxidative Stress: A Pilot Study, Antioxidants, doi:10.3390/antiox10122022
Wang, Xia, Tang, Wu, Zhu, A Novel Consistent Random Forest Framework: Bernoulli Random Forests, IEEE Trans. Neural Netw. Learn. Syst
Zhang, Wang, Shen, Zhang, Cen et al., Symptoms and Health Outcomes Among Survivors of COVID-19 Infection 1 Year After Discharge from Hospitals in Wuhan, China, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.27403
DOI record:
{
"DOI": "10.3390/ijms24087445",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms24087445",
"abstract": "<jats:p>In addition to the acute symptoms after infection, patients and society are also being challenged by the long-term effects of COVID-19, known as long COVID. Oxidative stress, as a pivotal point in the pathophysiology of COVID-19, could potentially be also involved in the development of the post-COVID syndrome. The aim of the present study was to evaluate the relationship between changes in oxidative status and the persistence of long-COVID symptoms in workers with a previous mild COVID-19 infection. A cross-sectional study was conducted among 127 employees of an Italian university (80 with a previous COVID-19 infection, and 47 healthy subjects). The TBARS assay was used to detect malondialdehyde serum levels (MDA), while total hydroperoxide (TH) production was measured by a d-ROMs kit. A significant difference in mean serum MDA values was found between previously infected subjects and healthy controls and (4.9 µm vs. 2.8 µm, respectively). Receiver–operating characteristic (ROC) curves showed high specificity and good sensibility (78.7% and 67.5%, respectively) for MDA serum levels. A random forest classifier identified the hematocrit value, MDA serum levels, and IgG titer against SARS-CoV-2 as features with the highest predictive value in distinguishing 34 long-COVID from 46 asymptomatic post-COVID subjects. Oxidative damage persists in subjects with previous COVID-19 infection, suggesting a possible role of oxidative stress mediators in the pathogenesis of long COVID.</jats:p>",
"alternative-id": [
"ijms24087445"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9101-7742",
"affiliation": [
{
"name": "Interdisciplinary Department of Medicine, University of Bari Aldo Moro, Piazza G. Cesare 11, 70124 Bari, Italy"
}
],
"authenticated-orcid": false,
"family": "Stufano",
"given": "Angela",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9981-4121",
"affiliation": [
{
"name": "Department of Translational Biomedicine Neuroscience, University of Bari Aldo Moro, Piazza G. Cesare 11, 70124 Bari, Italy"
}
],
"authenticated-orcid": false,
"family": "Isgrò",
"given": "Camilla",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3447-1535",
"affiliation": [
{
"name": "Department of Translational Biomedicine Neuroscience, University of Bari Aldo Moro, Piazza G. Cesare 11, 70124 Bari, Italy"
}
],
"authenticated-orcid": false,
"family": "Palese",
"given": "Luigi Leonardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Interdisciplinary Department of Medicine, University of Bari Aldo Moro, Piazza G. Cesare 11, 70124 Bari, Italy"
}
],
"family": "Caretta",
"given": "Paolo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0553-5309",
"affiliation": [
{
"name": "Interdisciplinary Department of Medicine, University of Bari Aldo Moro, Piazza G. Cesare 11, 70124 Bari, Italy"
}
],
"authenticated-orcid": false,
"family": "De Maria",
"given": "Luigi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1609-9397",
"affiliation": [
{
"name": "Interdisciplinary Department of Medicine, University of Bari Aldo Moro, Piazza G. Cesare 11, 70124 Bari, Italy"
}
],
"authenticated-orcid": false,
"family": "Lovreglio",
"given": "Piero",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8535-7601",
"affiliation": [
{
"name": "Department of Translational Biomedicine Neuroscience, University of Bari Aldo Moro, Piazza G. Cesare 11, 70124 Bari, Italy"
}
],
"authenticated-orcid": false,
"family": "Sardanelli",
"given": "Anna Maria",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
19
]
],
"date-time": "2023-04-19T05:39:05Z",
"timestamp": 1681882745000
},
"deposited": {
"date-parts": [
[
2023,
4,
19
]
],
"date-time": "2023-04-19T05:43:31Z",
"timestamp": 1681883011000
},
"indexed": {
"date-parts": [
[
2023,
4,
20
]
],
"date-time": "2023-04-20T05:56:45Z",
"timestamp": 1681970205047
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
4,
18
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T00:00:00Z",
"timestamp": 1681776000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/24/8/7445/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "7445",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
4,
18
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
18
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41586-021-03553-9",
"article-title": "High-dimensional characterization of post-acute sequelae of COVID-19",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "259",
"journal-title": "Nature",
"key": "ref_1",
"volume": "594",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103722",
"article-title": "Characterizing Long COVID: Deep Phenotype of a Complex Condition",
"author": "Deer",
"doi-asserted-by": "crossref",
"first-page": "103722",
"journal-title": "EBioMedicine",
"key": "ref_2",
"volume": "74",
"year": "2021"
},
{
"key": "ref_3",
"unstructured": "(2022, January 02). World Health Organization. Available online: WHO/2019-nCoV/Post_COVID-19_condition/Clinical_case_definition/2021."
},
{
"key": "ref_4",
"unstructured": "National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), and Royal College of General Practitioners (RCGP) (2022). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19, NICE. Available online: https://www.nice.org.uk/guidance/ng188/."
},
{
"DOI": "10.1038/s41598-021-95565-8",
"article-title": "More than 50 long-term effects of COVID-19: A systematic review and meta-analysis",
"author": "Perelman",
"doi-asserted-by": "crossref",
"first-page": "16144",
"journal-title": "Sci. Rep.",
"key": "ref_5",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.28568",
"article-title": "Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review",
"author": "Groff",
"doi-asserted-by": "crossref",
"first-page": "e2128568",
"journal-title": "JAMA Netw. Open.",
"key": "ref_6",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.3390/ijerph192416841",
"doi-asserted-by": "crossref",
"key": "ref_7",
"unstructured": "Goldhaber, N.H., Kohn, J.N., Ogan, W.S., Sitapati, A., Longhurst, C.A., Wang, A., Lee, S., Hong, S., and Horton, L.E. (2022). Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care. Int. J. Environ. Res. Public Health, 15."
},
{
"DOI": "10.1136/bmjopen-2020-048391",
"article-title": "COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study",
"author": "Dennis",
"doi-asserted-by": "crossref",
"first-page": "e048391",
"journal-title": "BMJ Open",
"key": "ref_8",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"article-title": "Post-acute COVID-19 syndrome",
"author": "Nalbandian",
"doi-asserted-by": "crossref",
"first-page": "601",
"journal-title": "Nat. Med.",
"key": "ref_9",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1186/s12967-022-03767-z",
"article-title": "Innate immunity, cytokine storm, and inflammatory cell death in COVID-19",
"author": "Karki",
"doi-asserted-by": "crossref",
"first-page": "542",
"journal-title": "J. Transl. Med.",
"key": "ref_10",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1155/2014/761264",
"article-title": "Oxidative stress, prooxidants, and antioxidants: The interplay",
"author": "Rahal",
"doi-asserted-by": "crossref",
"first-page": "761264",
"journal-title": "BioMed Res. Int.",
"key": "ref_11",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.3390/v10080392",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Khomich, O.A., Kochetkov, S.N., Bartosch, B., and Ivanov, A.V. (2018). Redox Biology of Respiratory Viral Infections. Viruses, 10."
},
{
"DOI": "10.1155/2014/360438",
"article-title": "Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal",
"author": "Ayala",
"doi-asserted-by": "crossref",
"first-page": "360438",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "ref_13",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1016/j.mehy.2020.110102",
"article-title": "SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression",
"author": "Cecchini",
"doi-asserted-by": "crossref",
"first-page": "110102",
"journal-title": "Med. Hypotheses",
"key": "ref_14",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)32656-8",
"article-title": "6-month consequences of COVID-19 in patients discharged from hospital: A cohort study",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "220",
"journal-title": "Lancet",
"key": "ref_15",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.3390/antiox10122022",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "van Eijk, L.E., Tami, A., Hillebrands, J.L., den Dunnen, W.F.A., de Borst, M.H., van der Voort, P.H.J., Bulthuis, M.L.C., Veloo, A.C.M., Wold, K.I., and Vincenti González, M.F. (2021). Mild Coronavirus Disease 2019 (COVID-19) Is Marked by Systemic Oxidative Stress: A Pilot Study. Antioxidants, 10."
},
{
"DOI": "10.1007/s10930-020-09935-8",
"article-title": "Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review",
"author": "Suhail",
"doi-asserted-by": "crossref",
"first-page": "644",
"journal-title": "Protein J.",
"key": "ref_17",
"volume": "39",
"year": "2020"
},
{
"article-title": "Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses",
"author": "Komaravelli",
"first-page": "1000141",
"journal-title": "J. Pharm. Pharm.",
"key": "ref_18",
"volume": "30",
"year": "2014"
},
{
"DOI": "10.1155/2016/9012580",
"article-title": "HCV and Oxidative Stress: Implications for HCV Life Cycle and HCV-Associated Pathogenesis",
"author": "Medvedev",
"doi-asserted-by": "crossref",
"first-page": "9012580",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "ref_19",
"volume": "2016",
"year": "2016"
},
{
"DOI": "10.1155/2016/8910396",
"article-title": "Oxidative Stress during HIV Infection: Mechanisms and Consequences",
"author": "Ivanov",
"doi-asserted-by": "crossref",
"first-page": "8910396",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "ref_20",
"volume": "2016",
"year": "2016"
},
{
"DOI": "10.1038/s41598-020-57914-x",
"article-title": "Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes",
"author": "Ledur",
"doi-asserted-by": "crossref",
"first-page": "1218",
"journal-title": "Sci. Rep.",
"key": "ref_21",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.4149/av_2014_03_253",
"article-title": "Enhanced oxidative damage to DNA, lipids, and proteins and levels of some antioxidant enzymes, cytokines, and heat shock proteins in patients infected with influenza H1N1 virus",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "253",
"journal-title": "Acta Virol.",
"key": "ref_22",
"volume": "58",
"year": "2014"
},
{
"DOI": "10.1038/s42255-022-00652-3",
"article-title": "Innate metabolic responses against viral infections",
"author": "Palmer",
"doi-asserted-by": "crossref",
"first-page": "1245",
"journal-title": "Nat. Metab.",
"key": "ref_23",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.isci.2021.103471",
"article-title": "Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis",
"author": "Medini",
"doi-asserted-by": "crossref",
"first-page": "103471",
"journal-title": "iScience",
"key": "ref_24",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"article-title": "Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages",
"author": "Merad",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_25",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1002/jmv.27526",
"article-title": "Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "1641",
"journal-title": "J. Med. Virol.",
"key": "ref_26",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.3390/antiox10071126",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Kosanovic, T., Sagic, D., Djukic, V., Pljesa-Ercegovac, M., Savic-Radojevic, A., Bukumiric, Z., Lalosevic, M., Djordjevic, M., Coric, V., and Simic, T. (2021). Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings. Antioxidants, 10."
},
{
"DOI": "10.1016/j.freeradbiomed.2021.01.044",
"article-title": "Susceptibility of the patients infected with Sars-Cov2 to oxidative stress and possible interplay with severity of the disease",
"author": "Gadotti",
"doi-asserted-by": "crossref",
"first-page": "184",
"journal-title": "Free Radic. Biol. Med.",
"key": "ref_28",
"volume": "165",
"year": "2021"
},
{
"DOI": "10.1016/j.arcmed.2021.06.004",
"article-title": "Changes in oxidative markers in COVID-19 patients",
"author": "Mehri",
"doi-asserted-by": "crossref",
"first-page": "843",
"journal-title": "Arch. Med. Res.",
"key": "ref_29",
"volume": "52",
"year": "2022"
},
{
"DOI": "10.3390/antiox11101958",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Higashi, Y. (2022). Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants, 11."
},
{
"DOI": "10.17113/ftb.52.04.14.3622",
"article-title": "Curcumin-Protected PC12 Cells Against Glutamate-Induced Oxidative Toxicity",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "468",
"journal-title": "Food Technol. Biotechnol.",
"key": "ref_31",
"volume": "52",
"year": "2014"
},
{
"DOI": "10.1111/j.1471-4159.2012.07934.x",
"article-title": "Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism",
"author": "Gonzalo",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "J. Neurochem.",
"key": "ref_32",
"volume": "123",
"year": "2012"
},
{
"DOI": "10.2217/bmm-2017-0420",
"article-title": "Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis",
"author": "Paliogiannis",
"doi-asserted-by": "crossref",
"first-page": "771",
"journal-title": "Biomark. Med.",
"key": "ref_33",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1016/j.cell.2017.09.021",
"article-title": "Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease",
"author": "Stockwell",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Cell",
"key": "ref_34",
"volume": "71",
"year": "2017"
},
{
"DOI": "10.1038/s41590-020-0699-0",
"article-title": "Redox homeostasis maintained by GPX4 facilitates STING activation",
"author": "Jia",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "Nat. Immunol.",
"key": "ref_35",
"volume": "21",
"year": "2020"
},
{
"article-title": "Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study",
"author": "Almulla",
"first-page": "1",
"journal-title": "Mol. Psychiatry",
"key": "ref_36",
"volume": "24",
"year": "2022"
},
{
"article-title": "Oxidative Stress Biomarkers and Their Association with Mortality among Patients Infected with SARS-CoV-2 in Mexico",
"author": "Lugo",
"first-page": "1058813",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "ref_37",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.redox.2021.102181",
"article-title": "Lipid peroxidation as a hallmark of severity in COVID-19 patients",
"author": "Aller",
"doi-asserted-by": "crossref",
"first-page": "102181",
"journal-title": "Redox Biol.",
"key": "ref_38",
"volume": "48",
"year": "2021"
},
{
"key": "ref_39",
"unstructured": "PHOSP-COVID Collaborative Group (2022). Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med., 10, 761–775."
},
{
"DOI": "10.1016/S2213-2600(22)00135-7",
"article-title": "Long COVID: Aiming for a consensus",
"author": "Munblit",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "Lancet Respir. Med.",
"key": "ref_40",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.27403",
"article-title": "Symptoms and Health Outcomes Among Survivors of COVID-19 Infection 1 Year After Discharge from Hospitals in Wuhan, China",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "e2127403",
"journal-title": "JAMA Netw. Open.",
"key": "ref_41",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1038/s41590-021-01113-x",
"article-title": "Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection",
"author": "Phetsouphanh",
"doi-asserted-by": "crossref",
"first-page": "210",
"journal-title": "Nat. Immunol.",
"key": "ref_42",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3390/jcm10194337",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Molnar, T., Varnai, R., Schranz, D., Zavori, L., Peterfi, Z., Sipos, D., Tőkés-Füzesi, M., Illes, Z., Buki, A., and Csecsei, P. (2021). Severe Fatigue and Memory Impairment Are Associated with Lower Serum Level of Anti-SARS-CoV-2 Antibodies in Patients with Post-COVID Symptoms. J. Clin. Med., 10."
},
{
"DOI": "10.1128/spectrum.02537-22",
"article-title": "Self-Reported Long COVID and Its Association with the Presence of SARS-CoV-2 Antibodies in a Danish Cohort up to 12 Months after Infection",
"author": "Larsen",
"doi-asserted-by": "crossref",
"first-page": "e0253722",
"journal-title": "Microbiol. Spectr.",
"key": "ref_44",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1155/2016/5698931",
"article-title": "Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox?",
"author": "Biswas",
"doi-asserted-by": "crossref",
"first-page": "5698931",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "ref_45",
"volume": "2016",
"year": "2016"
},
{
"DOI": "10.3390/antiox9111132",
"doi-asserted-by": "crossref",
"key": "ref_46",
"unstructured": "Jové, M., Mota-Martorell, N., Pradas, I., Martín-Gari, M., Ayala, V., and Pamplona, R. (2020). The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants, 9."
},
{
"DOI": "10.1038/s41577-020-0407-1",
"article-title": "Tissue damage from neutrophil-induced oxidative stress in COVID-19",
"author": "Laforge",
"doi-asserted-by": "crossref",
"first-page": "515",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_47",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1186/s12933-021-01359-7",
"article-title": "Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin",
"author": "Pretorius",
"doi-asserted-by": "crossref",
"first-page": "172",
"journal-title": "Cardiovasc. Diabetol.",
"key": "ref_48",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1093/brain/awaa240",
"article-title": "The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings",
"author": "Paterson",
"doi-asserted-by": "crossref",
"first-page": "3104",
"journal-title": "Brain",
"key": "ref_49",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1007/s00415-020-10380-x",
"article-title": "Central and peripheral nervous system complications of COVID-19: A prospective tertiary center cohort with 3-month follow-up",
"author": "Nersesjan",
"doi-asserted-by": "crossref",
"first-page": "3086",
"journal-title": "J. Neurol.",
"key": "ref_50",
"volume": "268",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2022.104211",
"article-title": "Anti-neuronal antibodies against brainstem antigens are associated with COVID-19",
"author": "Lucchese",
"doi-asserted-by": "crossref",
"first-page": "104211",
"journal-title": "EBioMedicine",
"key": "ref_51",
"volume": "83",
"year": "2022"
},
{
"article-title": "Brain neuronal and glialdamageduring acute COVID-19 infection in absence of clinical neurologicalmanifestations",
"author": "Plantone",
"first-page": "1343",
"journal-title": "J. Neurol. Neurosurg. Psychiatry",
"key": "ref_52",
"volume": "93",
"year": "2022"
},
{
"DOI": "10.1073/pnas.2024358118",
"article-title": "Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome",
"author": "Paul",
"doi-asserted-by": "crossref",
"first-page": "e2024358118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_53",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.3390/ijerph18094766",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Loconsole, D., Centrone, F., Morcavallo, C., Campanella, S., Sallustio, A., Accogli, M., Fortunato, F., Parisi, A., and Chironna, M. (2021). Rapid Spread of the SARS-CoV-2 Variant of Concern 202012/01 in Southern Italy (December 2020–March 2021). Int. J. Environ. Res. Public Health, 18."
},
{
"key": "ref_55",
"unstructured": "COVID-19 Treatment Guidelines Panel (2022, April 02). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, Available online: https://www.covid19treatmentguidelines.nih.gov/."
},
{
"DOI": "10.1038/s41598-022-15357-6",
"article-title": "Impact of COVID-19 emergency on the psychological well-being of susceptible individuals",
"author": "Stufano",
"doi-asserted-by": "crossref",
"first-page": "11152",
"journal-title": "Sci. Rep.",
"key": "ref_56",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1203/00006450-200002000-00012",
"article-title": "Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies",
"author": "Buonocore",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Pediatr. Res.",
"key": "ref_57",
"volume": "47",
"year": "2000"
},
{
"DOI": "10.3390/ijms232112774",
"doi-asserted-by": "crossref",
"key": "ref_58",
"unstructured": "Isgrò, C., Spagnuolo, L., Pannucci, E., Mondello, L., Santi, L., Dugo, L., and Sardanelli, A.M. (2022). Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson’s Disease Patients and THP-1 Macrophages. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1109/TNNLS.2017.2729778",
"article-title": "A Novel Consistent Random Forest Framework: Bernoulli Random Forests",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "3510",
"journal-title": "IEEE Trans. Neural Netw. Learn. Syst.",
"key": "ref_59",
"volume": "29",
"year": "2018"
}
],
"reference-count": 59,
"references-count": 59,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/24/8/7445"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Inorganic Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Computer Science Applications",
"Spectroscopy",
"Molecular Biology",
"General Medicine",
"Catalysis"
],
"subtitle": [],
"title": "Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers",
"type": "journal-article",
"volume": "24"
}