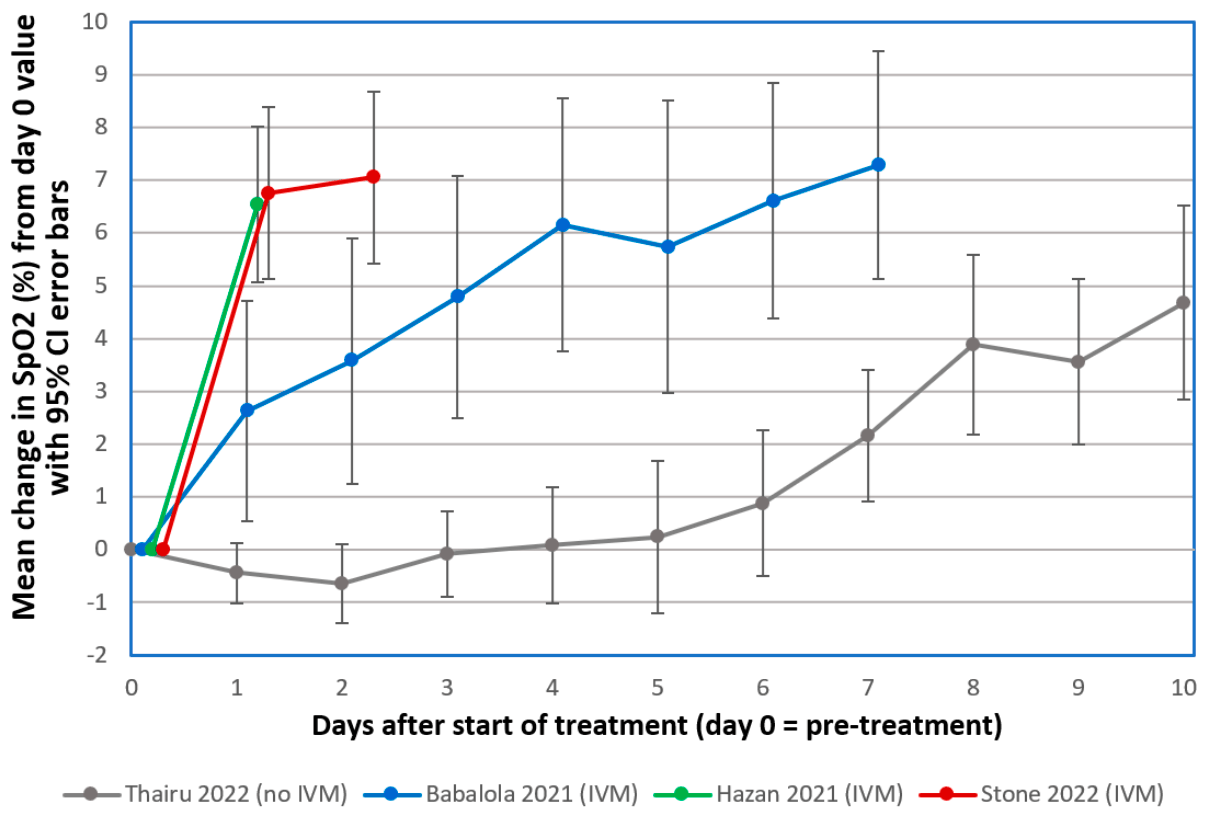
Changes in SpO2 on Room Air for 34 Severe COVID-19 Patients after Ivermectin-Based Combination Treatment: 62% Normalization within 24 Hours
et al., Biologics, doi:10.3390/biologics2030015, Nov 2021 (preprint)
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000028 from 47 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective severe COVID-19 patients in Zimbabwe treated with ivermectin, doxycycline, and zinc. For 34 with SpO2 tracking, there was rapid improvement in SpO2, with 55% recovery towards SpO2 97 within 12 hours. The preprint shows further analysis of all 92 severe cases: there were 2 deaths and 2 additional progressions prior to recovery, significantly less than a predicted 7 deaths and 17 deteriorations based on demographics and risk factors1.
Study covers ivermectin and zinc.
Stone et al., 9 Nov 2021, retrospective, South Africa, peer-reviewed, mean age 55.0, 7 authors, study period August 2020 - May 2021.
Contact: dscheim@alum.mit.edu (corresponding author).
Changes in SpO2 on Room Air for 34 Severe COVID-19 Patients after Ivermectin-Based Combination Treatment: 62% Normalization within 24 Hours
Biologics, doi:10.3390/biologics2030015
The emergence of COVID-19 in March 2020 challenged Zimbabwe to respond with limited medical facilities and therapeutic options. Based on early clinical indications of efficacy for the macrocyclic lactone, Ivermectin (IVM), against COVID-19, IVM-based combination treatments were deployed to treat it. Oxygen saturation (SpO2) data were retrospectively analyzed for 34 severe, hypoxic COVID-19 patients all on room air (without supplemental oxygen). The patients, median age 56.5, were treated at clinics or at home between August 2020 and May 2021. All but three of these 34 patients had significantly increased SpO2 values within 24 h after the first IVM dose. The mean increase in SpO2 as a percentage of full normalization to SpO2 = 97 was 55.1% at +12 h and 62.3% at +24 h after the first IVM dose (paired t-test, p < 0.0000001). These results parallel similar sharp, rapid increases in SpO2, all on room air, for 24 mostly severe COVID-19 patients in the USA (California) who were given an IVM-based combination treatment. All patients in both of these critical series recovered. These rapid increases in SpO2 values after IVM treatment stand in sharp contrast to declines in SpO2 and associated pulmonary function through the second week following the onset of moderate or severe COVID-19 symptoms under standard care.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Abbreviations The
References
Aminpour, Cannariato, Safaeeardebili, Preto, Moracchiato et al., In silico analysis of the multi-targeted mode of action of ivermectin and related compounds, Computation, doi:10.3390/computation10040051
Annunziata, Coppola, Carannante, Simioli, Lanza et al., Home management of patients with moderate or severe respiratory failure secondary to COVID-19, using remote monitoring and oxygen with or without HFNC, Pathogens, doi:10.3390/pathogens10040413
Aoki, Iwasawa, Hagiwara, Komatsu, Utsunomiya et al., Pulmonary vascular enlargement and lesion extent on computed tomography are correlated with COVID-19 disease severity, Jpn. J. Radiol, doi:10.1007/s11604-020-01085-2
Arévalo, Pagotto, Pórfido, Daghero, Segovia et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Sci. Rep
Ayerbe, Risco, Ayis, The association between treatment with heparin and survival in patients with Covid-19, J. Thromb. Thrombolysis, doi:10.1007/s11239-020-02162-z
Babalola, Karu, Personal communication
Babalola, Ndanusa, Adesuyi, Ogedengbe, Thairu et al., A randomized controlled trial of ivermectin monotherapy versus HCQ, IVM, and AZ combination therapy in COVID-19 patients in Nigeria, J. Infect. Dis. Epidemiol, doi:10.23937/2474-3658/1510233
Campbell, History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents, Curr. Pharm. Biotechnol, doi:10.2174/138920112800399095
Chirisa, Zimbabwe Confirms Its First Case of Coronavirus
Chung, Yang, Wu, Deng, Tsai, Agricultural avermectins: An uncommon but potentially fatal cause of pesticide poisoning, Ann. Emerg. Med, doi:10.1016/S0196-0644(99)70271-4
Crump, Ōmura, Ivermectin, 'wonder drug' from Japan: The human use perspective, Proc. Jpn. Acad. Ser. B Phys. Biol. Sci, doi:10.2183/pjab.87.13
Dayer, Coronavirus (2019-nCoV) deactivation via spike glycoprotein shielding by old drugs, bioinformatic study, doi:10.20944/preprints202005.0020.v1
De Castro, Jr, Gregianin, Burger, Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection, Leuk Lymphoma
De Melo, Lazarini, Levallois, Hautefort, Michel et al., COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters, Sci. Transl. Med
Ding, Xu, Zhou, Long, Chest CT findings of COVID-19 pneumonia by duration of symptoms, Eur. J. Radiol, doi:10.1016/j.ejrad.2020.109009
George, Borody, Andrews, Devine, Moore-Jones et al., Cure of duodenal ulcer after eradication of Helicobacter pylori, Med. J. Aust, doi:10.5694/j.1326-5377.1990.tb136833.x
Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J. Clin. Pharmacol, doi:10.1177/009127002401382731
Hashim, Maulood, Rasheed, Fatak, Kabah et al., Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq, doi:10.22578/IJMS.19.1.14
Hazan, Dave, Gunaratne, Dolai, Clancy et al., Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients, Future Microbiol, doi:10.2217/fmb-2022-0014
Hill, Garratt, Levi, Falconer, Ellis et al., Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection, Open Forum Infect. Dis, doi:10.1093/ofid/ofab358
Kory, Meduri, Varon, Iglesias, Marik, Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19, Am. J. Ther, doi:10.1097/MJT.0000000000001377
Krause, Buisson, Bertrand, Corringer, Galzi et al., Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor, Mol. Pharmacol, doi:10.1124/mol.53.2.283
Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100959
Lim, Hor, Tay, Mat, Jelani et al., Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: The I-TECH randomized clinical trial, JAMA Intern. Med, doi:10.1001/jamainternmed.2022.0189
López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2021.3071
Mahmud, Rahman, Alam, Ahmed, Kabir et al., Ivermectin in combination with doxycycline for treating COVID-19 symptoms: A randomized trial, J. Int. Med. Res, doi:10.1177/03000605211013550
Metwally, Basha, Zaitoun, Abdalla, Nofal et al., Clinical and radiological imaging as prognostic predictors in COVID-19 patients, J. Radiol. Nucl. Med, doi:10.1186/s43055-021-00470-9
Navarro, Camprubí, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: A systematic review and meta-analysis, J. Antimicrob. Chemother
Negri, Piloto, Morinaga, Jardim, Lamy et al., Heparin therapy improving hypoxia in COVID-19 patients-A case series, Front. Physiol, doi:10.3389/fphys.2020.573044
Osman, Farouk, Osman, Abdrabou, Longitudinal assessment of chest computerized tomography and oxygen saturation for patients with COVID-19. Egypt, J. Radiol. Nucl. Med, doi:10.1186/s43055-020-00376-y
Quispe-Cholan, Anticona-De-La-Cruz, Cornejo-Cruz, Quispe-Chirinos, Moreno-Lazaro et al., Tomographic findings in patients with COVID-19 according to evolution of the disease, J. Radiol. Nucl. Med
Rajter, None
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19 (ICON study), Chest, doi:10.1016/j.chest.2020.10.009
Reis, Silva, Silva, Thabane, Milagres et al., Effect of early treatment with ivermectin among patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2115869
Ren, Tong, Li, Lu, Yao, The protective effect of alpha 7 nicotinic acetylcholine receptor activation on critical illness and its mechanism, Int. J. Biol. Sci, doi:10.7150/ijbs.16404
Santin, Scheim, Mccullough, Yagisawa, Borody, Ivermectin: A multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, N. Microbes N. Infect, doi:10.1016/j.nmni.2021.100924
Scheim, A deadly embrace: Hemagglutination mediated by SARS-CoV-2 spike protein at its 22 N-glycosylation sites, red blood cell surface sialoglycoproteins, and antibody, Int. J. Mol. Sci, doi:10.3390/ijms23052558
Scheim, Hibberd, Chamie-Quintero, Protocol Violations in López-Medina et al.: 38 Switched Ivermectin (IVM) and Placebo Doses, Failure of Blinding, doi:10.31219/osf.io/u7ewz
Scheim, The Drug Used in Lim et al, doi:10.31219/osf.io/5cwmr
Stone, Ndarukwa, Scheim, Dancis, Dancis et al., Rapid increase of SpO 2 on room air for 34 severe COVID-19 patients after ivermectin-based combination treatment: 55-62% normalization within, Res. Sq
Thairu, Babalola, Ajayi, Ndanusa, Ogedengbe et al., A comparison of Ivermectin and non Ivermectin based regimen for COVID-19 in Abuja: Effects on virus clearance, days-to-discharge and mortality, Res. Sq, doi:10.9734/jpri/2022/v34i44A36328
Wang, Dong, Hu, Li, Ren et al., Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study, Radiology, doi:10.1148/radiol.2020200843
Wang, Yu, Ochani, Amella, Tanovic et al., Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation, Nature, doi:10.1038/nature01339
Who, COVID-19)
Yagisawa, Foster, Hanaki, Omura, Global trends in clinical studies of ivermectin in COVID-19, Jpn. J. Antibiot
Yin, Huang, Li, Tang, Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2, J. Thromb. Thrombolysis, doi:10.1007/s11239-020-02105-8
Zaidi, Dehgani-Mobaraki, The mechanisms of action of ivermectin against SARS-CoV-2-An extensive review, J. Antibiot, doi:10.1038/s41429-021-00491-6
DOI record:
{
"DOI": "10.3390/biologics2030015",
"ISSN": [
"2673-8449"
],
"URL": "http://dx.doi.org/10.3390/biologics2030015",
"abstract": "<jats:p>The emergence of COVID-19 in March 2020 challenged Zimbabwe to respond with limited medical facilities and therapeutic options. Based on early clinical indications of efficacy for the macrocyclic lactone, Ivermectin (IVM), against COVID-19, IVM-based combination treatments were deployed to treat it. Oxygen saturation (SpO2) data were retrospectively analyzed for 34 severe, hypoxic COVID-19 patients all on room air (without supplemental oxygen). The patients, median age 56.5, were treated at clinics or at home between August 2020 and May 2021. All but three of these 34 patients had significantly increased SpO2 values within 24 h after the first IVM dose. The mean increase in SpO2 as a percentage of full normalization to SpO2 = 97 was 55.1% at +12 h and 62.3% at +24 h after the first IVM dose (paired t-test, p < 0.0000001). These results parallel similar sharp, rapid increases in SpO2, all on room air, for 24 mostly severe COVID-19 patients in the USA (California) who were given an IVM-based combination treatment. All patients in both of these critical series recovered. These rapid increases in SpO2 values after IVM treatment stand in sharp contrast to declines in SpO2 and associated pulmonary function through the second week following the onset of moderate or severe COVID-19 symptoms under standard care.</jats:p>",
"alternative-id": [
"biologics2030015"
],
"author": [
{
"affiliation": [],
"family": "Stone",
"given": "Jaqueline C.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-6815-8088",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ndarukwa",
"given": "Pisirai",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6841-7054",
"affiliation": [],
"authenticated-orcid": false,
"family": "Scheim",
"given": "David E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1498-4042",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dancis",
"given": "Barry M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dancis",
"given": "Jerome",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gill",
"given": "Martin G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aldous",
"given": "Colleen",
"sequence": "additional"
}
],
"container-title": "Biologics",
"container-title-short": "Biologics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
1
]
],
"date-time": "2022-09-01T03:53:21Z",
"timestamp": 1662004401000
},
"deposited": {
"date-parts": [
[
2022,
9,
1
]
],
"date-time": "2022-09-01T04:23:59Z",
"timestamp": 1662006239000
},
"indexed": {
"date-parts": [
[
2022,
9,
1
]
],
"date-time": "2022-09-01T04:51:53Z",
"timestamp": 1662007913312
},
"is-referenced-by-count": 1,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
8,
31
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
31
]
],
"date-time": "2022-08-31T00:00:00Z",
"timestamp": 1661904000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2673-8449/2/3/15/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "196-210",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
8,
31
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
31
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "Worldometer Coronovirus Statistics\nhttps://www.worldometers.info/coronavirus/#countries"
},
{
"article-title": "Global trends in clinical studies of ivermectin in COVID-19",
"author": "Yagisawa",
"first-page": "44",
"journal-title": "Jpn. J. Antibiot.",
"key": "ref2",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1097/MJT.0000000000001377",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1093/ofid/ofab358",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.nmni.2021.100924",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1056/NEJMoa2115869",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"author": "U.S. National Institutes of Health",
"key": "ref9",
"series-title": "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines: Therapeutic Management of Patients with COVID-19",
"year": "2021"
},
{
"key": "ref10",
"series-title": "WHO Recommends against the Use of Remdesivir in COVID-19 Patients",
"year": "2020"
},
{
"DOI": "10.3390/computation10040051",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3390/ijms23052558",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"key": "ref13",
"unstructured": "Zimbabwe Confirms Its First Case of Coronavirus, 20 March 2020\nhttps://iharare.com/zimbabwe-confirms-first-case-of-coronavirus-2/"
},
{
"key": "ref14",
"series-title": "Coronavirus Disease 2019 (COVID-19), Situation Report—72",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.2174/138920112800399095",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.2183/pjab.87.13",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1177/009127002401382731",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1093/jac/dkz524",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1080/10428194.2020.1786559",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/S0196-0644(99)70271-4",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.31219/osf.io/u7ewz",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.31219/osf.io/5cwmr",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1126/scitranslmed.abf8396",
"article-title": "COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters",
"author": "de Melo",
"doi-asserted-by": "crossref",
"first-page": "eabf8396",
"journal-title": "Sci. Transl. Med.",
"key": "ref25",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-86679-0",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1177/03000605211013550",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.22578/IJMS.19.1.14",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.5694/j.1326-5377.1990.tb136833.x",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"article-title": "Coronavirus Disease 2019 (COVID-19). The Basics of Oxygen Monitoring and Oxygen Therapy during the COVID-19 Pandemic",
"author": "U.S. Centers for Disease Control and Prevention (CDC)",
"key": "ref30"
},
{
"DOI": "10.1186/s43055-020-00376-y",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1186/s43055-021-00470-9",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1007/s11604-020-01085-2",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/j.ejrad.2020.109009",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1148/radiol.2020200843",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1186/s43055-020-00329-5",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3390/pathogens10040413",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.9734/jpri/2022/v34i44A36328",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.23937/2474-3658/1510233",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1007/s11239-020-02162-z",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.3389/fphys.2020.573044",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1007/s11239-020-02105-8",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.20944/preprints202005.0020.v1",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"author": "Babalola",
"journal-title": "Personal communication",
"key": "ref44",
"year": "2022"
},
{
"DOI": "10.2217/fmb-2022-0014",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"author": "Rajter",
"journal-title": "Personal communication",
"key": "ref46",
"year": "2020"
},
{
"DOI": "10.21203/rs.3.rs-1048271/v1",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1038/s41429-021-00491-6",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1038/nature01339",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1124/mol.53.2.283",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.7150/ijbs.16404",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"article-title": "Clinical Spectrum of SARS-CoV-2 Infection",
"author": "U.S. National Institutes of Health (NIH)",
"key": "ref52"
},
{
"key": "ref53",
"series-title": "The 2015 Nobel Prize in Physiology or Medicine—Press Release",
"year": "2015"
}
],
"reference-count": 53,
"references-count": 53,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2673-8449/2/3/15"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Earth and Planetary Sciences",
"General Environmental Science"
],
"subtitle": [],
"title": "Changes in SpO2 on Room Air for 34 Severe COVID-19 Patients after Ivermectin-Based Combination Treatment: 62% Normalization within 24 Hours",
"type": "journal-article",
"volume": "2"
}
stone
