Effect of vitamin A supplementation on the outcome severity of COVID-19 in hospitalized patients: A pilot randomized clinical trial
et al., Nutrition and Health, doi:10.1177/02601060221129144, IRCT20170117032004N3, Oct 2022
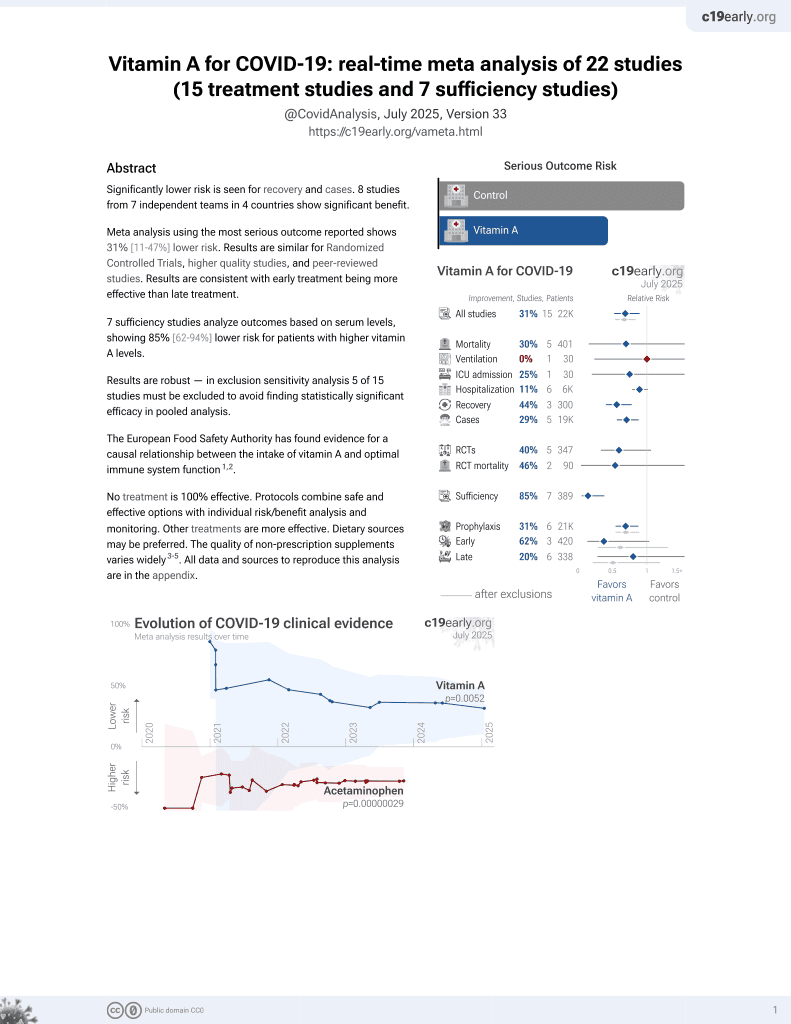
Vitamin A for COVID-19
49th treatment shown to reduce risk in
May 2023, now with p = 0.004 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 30 hospitalized patients in Iran, showing no significant difference with vitamin A treatment. All patients received HCQ. 50,000 IU/day intramuscular vitamin A for up to 2 weeks.
There are multiple potential data issues:
- With the reported values, the lymphocyte count p-value appears off by ~50x, should be <0.02
- The overall age SD is inconsistent with the group age SDs
- The control group's age SD of 4.94 is very low. In a randomized trial with n=30, such dramatically different variances are highly improbable.
- The control group mean age of 61.28 x 15 = 919.2, which is not an integer. For whole-year ages, the sum should be an integer (it is for the treatment group). Several values fail the GRIM test if coded as integers: control age, both time to response values, control hospital stay.
- The O2 saturation IQR differs by a factor of 4.4 between groups, unexpected for randomized allocation.
- The serum creatinine, cough, and myalgia p-values are inconsistent with the data.
- “Placebo” is used in some places and “control” in others. The abstract suggests no placebo: “Patients in the control group continued their common treatment protocols”, and authors note neither physicians nor patients were blind to their group.
- Table 1 indicates chi-square for continuous variables like temperature and heart rate.
- Unclear how deaths are treated for time to clinical response.
- Reports ESR as erythropoietin sedimentation rate; likely meant to be erythrocyte sedimentation rate.
- 9 patients were excluded with no reasons provided.
- The overall age SD is inconsistent with the group age SDs
- The control group's age SD of 4.94 is very low. In a randomized trial with n=30, such dramatically different variances are highly improbable.
- The control group mean age of 61.28 x 15 = 919.2, which is not an integer. For whole-year ages, the sum should be an integer (it is for the treatment group). Several values fail the GRIM test if coded as integers: control age, both time to response values, control hospital stay.
- The O2 saturation IQR differs by a factor of 4.4 between groups, unexpected for randomized allocation.
- The serum creatinine, cough, and myalgia p-values are inconsistent with the data.
- “Placebo” is used in some places and “control” in others. The abstract suggests no placebo: “Patients in the control group continued their common treatment protocols”, and authors note neither physicians nor patients were blind to their group.
- Table 1 indicates chi-square for continuous variables like temperature and heart rate.
- Unclear how deaths are treated for time to clinical response.
- Reports ESR as erythropoietin sedimentation rate; likely meant to be erythrocyte sedimentation rate.
- 9 patients were excluded with no reasons provided.
This study is excluded in meta-analysis:
multiple data issues - pending author response.
|
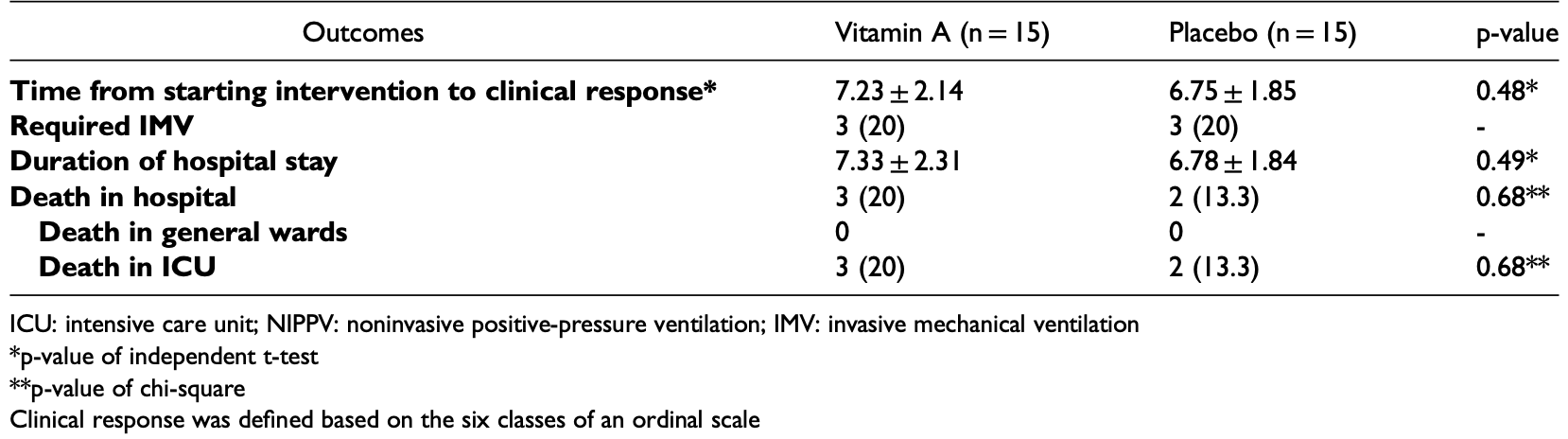
risk of death, 50.0% higher, RR 1.50, p = 1.00, treatment 3 of 15 (20.0%), control 2 of 15 (13.3%).
|
|
risk of mechanical ventilation, no change, RR 1.00, p = 1.00, treatment 3 of 15 (20.0%), control 3 of 15 (20.0%).
|
|
risk of ICU admission, 25.0% lower, RR 0.75, p = 1.00, treatment 3 of 15 (20.0%), control 4 of 15 (26.7%), NNT 15.
|
|
time to clinical response, 76.0% higher, HR 1.76, p = 0.21, treatment 15, control 15, Kaplan-Meier.
|
|
hospitalization time, 8.1% higher, relative time 1.08, p = 0.49, treatment mean 7.33 (±2.31) n=15, control mean 6.78 (±1.84) n=15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Somi et al., 7 Oct 2022, Randomized Controlled Trial, Iran, peer-reviewed, mean age 60.2, 7 authors, study period April 2020 - July 2020, trial IRCT20170117032004N3.
Contact: znikniaz@hotmail.com.
Effect of vitamin A supplementation on the outcome severity of COVID-19 in hospitalized patients: A pilot randomized clinical trial
Nutrition and Health, doi:10.1177/02601060221129144
Introduction: Vitamin A is one of the vitamins that is suggested as adjuvant therapy in viral infections due to its immune enhancing role. In the present clinical trial, we intended to assess the effect of vitamin A supplementation on Coronavirus disease-2019 (COVID-19) in hospitalized patients. Methods: The present pilot randomized controlled clinical trial was conducted on 30 hospitalized patients with COVID-19. Patients in the intervention group received 50000 IU/day intramuscular vitamin A for a maximum of two weeks. Patients in the control group continued their common treatment protocols. All participants were followed up until discharge from the hospital or death. The primary outcome of the study was time to achieve clinical response based on the six classes of an ordinal scale. Time to clinical response was calculated based on the days needed to improve two scores on the scale or patient's discharge. Results: The time to clinical response was not significantly different between the two groups (7.23 ± 2.14 vs. 6.75 ± 1.85 days, respectively, p = 0.48). There was no significant difference between the groups regarding clinical response (hazard ratio: 1.76 [95% CI: 0.73, 4.26]). There were no significant differences between groups regarding the need for mechanical ventilation, duration of hospitalization, or death in the hospital. Conclusion: The results of this pilot clinical trial showed no benefit of vitamin A compared with the common treatment on outcome severity in hospitalized patients with COVID-19. Although the results are negative, there is still a great need for future clinical studies to provide a higher level of evidence.
Declaration of conflicting interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee of Tabriz University of Medical Sciences (Ethics code: IR.TBZMED.REC.1398.1305). Written informed consent was obtained from all participants. In addition, the protocol of the trial was registered in IRCT (IRCT20170117032004N3).
References
Carr, A new clinical trial to test high-dose vitamin C in patients with COVID-19, Critical Care
Cucinotta, Vanelli, WHO Declares COVID-19 a pandemic, Acta bio-medica: Atenei Parmensis
Esakandari, Nabi-Afjadi, Fakkari-Afjadi, A comprehensive review of COVID-19 characteristics, Biological Procedures Online
Fawzi, Mbise, Fataki, Vitamin A supplementation and severity of pneumonia in children admitted to the hospital in Dar es Salaam, Tanzania, The American Journal of Clinical Nutrition
Grotto, Mimouni, Gdalevich, Vitamin A supplementation and childhood morbidity from diarrhea and respiratory infections: A meta-analysis, The Journal of Pediatrics
Iddir, Brito, Dingeo, Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis, Nutrients
Li, Wu, Li, Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19, Aging
Madatuwa, Mahawithanage, Chandrika, Evaluation of the effectiveness of the national vitamin A supplementation programme among school children in Sri Lanka, British Journal of Nutrition
Martins-Filho, Tavares, Vs, Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data, European Journal Of Internal Medicine
Nacul, Arthur, Kirkwood, The impact of vitamin A supplementation given during a pneumonia episode on the subsequent morbidity of children, Tropical Medicine & International Health
Peterson, Vock, Powers, Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization, Clinical Trials
Rodríguez, Hamer, Rivera, Effects of moderate doses of vitamin A as an adjunct to the treatment of pneumonia in underweight and normal-weight children: A randomized, double-blind, placebo-controlled trial, The American Journal Of Clinical Nutrition
Said, Mousa, Fawzi, Combined effect of high-dose vitamin A, vitamin E supplementation, and zinc on adult patients with diabetes: A randomized trial, Journal of Advanced Research
Si, Grytter, Vy, High dose vitamin A supplementation in the course of pneumonia in Vietnamese children, Acta Paediatrica
Soekarjo, Sd, Kusin, Effectiveness of weekly vitamin A (10,000 IU) and iron (60 mg) supplementation for adolescent boys and girls through schools in rural and urban east Java, Indonesia, European Journal of Clinical Nutrition
Sommer, Rahmathullah, Underwood, Potential interventions for the prevention of childhood pneumonia in developing countries: A meta-analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality. The vitamin A and pneumonia working group, Bulletin of the World Health Organization
Stephensen, Vitamin A, infection, and immune function, Annual Review Of Nutrition
Whitehead, Julious, Cooper, Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable, Statistical Methods In Medical Research
Wu, Ni, Wei, Vitamin A for non-measles pneumonia in children, Cochrane Database of Systematic Reviews
Zhang, Niu, ACE2 and COVID-19 and the resulting ARDS, Postgraduate Medical Journal
Zhou, Zuo, Li, Effects of nutrition intervention on the nutritional status and outcomes of pediatric patients with pneumonia, Minerva Pediatrica
DOI record:
{
"DOI": "10.1177/02601060221129144",
"ISSN": [
"0260-1060",
"2047-945X"
],
"URL": "http://dx.doi.org/10.1177/02601060221129144",
"abstract": "<jats:sec><jats:title>Introduction</jats:title><jats:p> Vitamin A is one of the vitamins that is suggested as adjuvant therapy in viral infections due to its immune enhancing role. In the present clinical trial, we intended to assess the effect of vitamin A supplementation on Coronavirus disease-2019 (COVID-19) in hospitalized patients. </jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p> The present pilot randomized controlled clinical trial was conducted on 30 hospitalized patients with COVID-19. Patients in the intervention group received 50000 IU/day intramuscular vitamin A for a maximum of two weeks. Patients in the control group continued their common treatment protocols. All participants were followed up until discharge from the hospital or death. The primary outcome of the study was time to achieve clinical response based on the six classes of an ordinal scale. Time to clinical response was calculated based on the days needed to improve two scores on the scale or patient's discharge. </jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p> The time to clinical response was not significantly different between the two groups (7.23 ± 2.14 vs. 6.75 ± 1.85 days, respectively, p = 0.48). There was no significant difference between the groups regarding clinical response (hazard ratio: 1.76 [95% CI: 0.73, 4.26]). There were no significant differences between groups regarding the need for mechanical ventilation, duration of hospitalization, or death in the hospital. </jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p> The results of this pilot clinical trial showed no benefit of vitamin A compared with the common treatment on outcome severity in hospitalized patients with COVID-19. Although the results are negative, there is still a great need for future clinical studies to provide a higher level of evidence. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/02601060221129144"
],
"author": [
{
"affiliation": [
{
"name": "Gastroenterologist, Liver and gastrointestinal diseases research center, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"family": "Somi",
"given": "Mohammad Hossein",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Gastroenterologist, Liver and gastrointestinal diseases research center, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"family": "Faghih Dinevari",
"given": "Masood",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pulmonologist, Tuberculosis and Lung Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"family": "Taghizadieh",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious disease specialist, Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"family": "Varshochi",
"given": "Mojtaba",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"family": "Sadeghi Majd",
"given": "Elham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internist, Liver and gastrointestinal diseases research center, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"family": "Abbasian",
"given": "Samaneh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6522-1048",
"affiliation": [
{
"name": "Gastroenterologist, Liver and gastrointestinal diseases research center, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"authenticated-orcid": false,
"family": "Nikniaz",
"given": "Zeinab",
"sequence": "additional"
}
],
"container-title": "Nutrition and Health",
"container-title-short": "Nutr Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
7
]
],
"date-time": "2022-10-07T08:23:26Z",
"timestamp": 1665131006000
},
"deposited": {
"date-parts": [
[
2022,
10,
7
]
],
"date-time": "2022-10-07T08:23:33Z",
"timestamp": 1665131013000
},
"funder": [
{
"name": "Liver and gastrointestinal diseases research center, Tabriz University of Medical Sciences, Tabriz, Iran"
}
],
"indexed": {
"date-parts": [
[
2022,
10,
7
]
],
"date-time": "2022-10-07T08:44:55Z",
"timestamp": 1665132295663
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
7
]
]
},
"language": "en",
"license": [
{
"URL": "http://journals.sagepub.com/page/policies/text-and-data-mining-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
7
]
],
"date-time": "2022-10-07T00:00:00Z",
"timestamp": 1665100800000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/02601060221129144",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/02601060221129144",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/02601060221129144",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "026010602211291",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2022,
10,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
7
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1186/s13054-020-02851-4",
"doi-asserted-by": "publisher",
"key": "bibr1-02601060221129144"
},
{
"author": "Cucinotta D",
"first-page": "157",
"journal-title": "Acta bio-medica: Atenei Parmensis",
"key": "bibr2-02601060221129144",
"volume": "91",
"year": "2020"
},
{
"DOI": "10.1186/s12575-020-00128-2",
"doi-asserted-by": "publisher",
"key": "bibr3-02601060221129144"
},
{
"DOI": "10.1093/ajcn/68.1.187",
"doi-asserted-by": "publisher",
"key": "bibr4-02601060221129144"
},
{
"DOI": "10.1067/mpd.2003.116",
"doi-asserted-by": "publisher",
"key": "bibr5-02601060221129144"
},
{
"DOI": "10.3390/nu12061562",
"doi-asserted-by": "publisher",
"key": "bibr6-02601060221129144"
},
{
"DOI": "10.18632/aging.103888",
"doi-asserted-by": "publisher",
"key": "bibr7-02601060221129144"
},
{
"DOI": "10.1017/S0007114507191923",
"doi-asserted-by": "publisher",
"key": "bibr8-02601060221129144"
},
{
"DOI": "10.1016/j.ejim.2020.04.043",
"doi-asserted-by": "publisher",
"key": "bibr9-02601060221129144"
},
{
"DOI": "10.1046/j.1365-3156.1998.00259.x",
"doi-asserted-by": "publisher",
"key": "bibr10-02601060221129144"
},
{
"DOI": "10.1177/1740774517697919",
"doi-asserted-by": "publisher",
"key": "bibr11-02601060221129144"
},
{
"key": "bibr12-02601060221129144",
"unstructured": "Physicians RCo. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. London: RCP, 2017. Available at: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2."
},
{
"DOI": "10.1093/ajcn/82.5.1090",
"doi-asserted-by": "publisher",
"key": "bibr13-02601060221129144"
},
{
"author": "Said E",
"journal-title": "Journal of Advanced Research",
"key": "bibr14-02601060221129144",
"year": "2020"
},
{
"DOI": "10.1111/j.1651-2227.1997.tb14805.x",
"doi-asserted-by": "publisher",
"key": "bibr15-02601060221129144"
},
{
"DOI": "10.1038/sj.ejcn.1601914",
"doi-asserted-by": "publisher",
"key": "bibr16-02601060221129144"
},
{
"author": "Sommer A",
"first-page": "609",
"journal-title": "Bulletin of the World Health Organization",
"key": "bibr17-02601060221129144",
"volume": "73",
"year": "1995"
},
{
"DOI": "10.1146/annurev.nutr.21.1.167",
"doi-asserted-by": "publisher",
"key": "bibr18-02601060221129144"
},
{
"DOI": "10.1177/0962280215588241",
"doi-asserted-by": "publisher",
"key": "bibr19-02601060221129144"
},
{
"DOI": "10.1002/14651858.CD003700.pub2",
"doi-asserted-by": "publisher",
"key": "bibr20-02601060221129144"
},
{
"DOI": "10.1136/postgradmedj-2020-137935",
"doi-asserted-by": "publisher",
"key": "bibr21-02601060221129144"
},
{
"author": "Zhou W",
"first-page": "5",
"journal-title": "Minerva Pediatrica",
"key": "bibr22-02601060221129144",
"volume": "68",
"year": "2016"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/02601060221129144"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Nutrition and Dietetics",
"General Medicine",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "Effect of vitamin A supplementation on the outcome severity of COVID-19 in hospitalized patients: A pilot randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy"
}