The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID‐19‐ related cough: A randomized, controlled clinical trial
et al., The Clinical Respiratory Journal, doi:10.1111/crj.13529, Jul 2022
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0041 from 9 studies.
Lower risk for hospitalization and cases.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
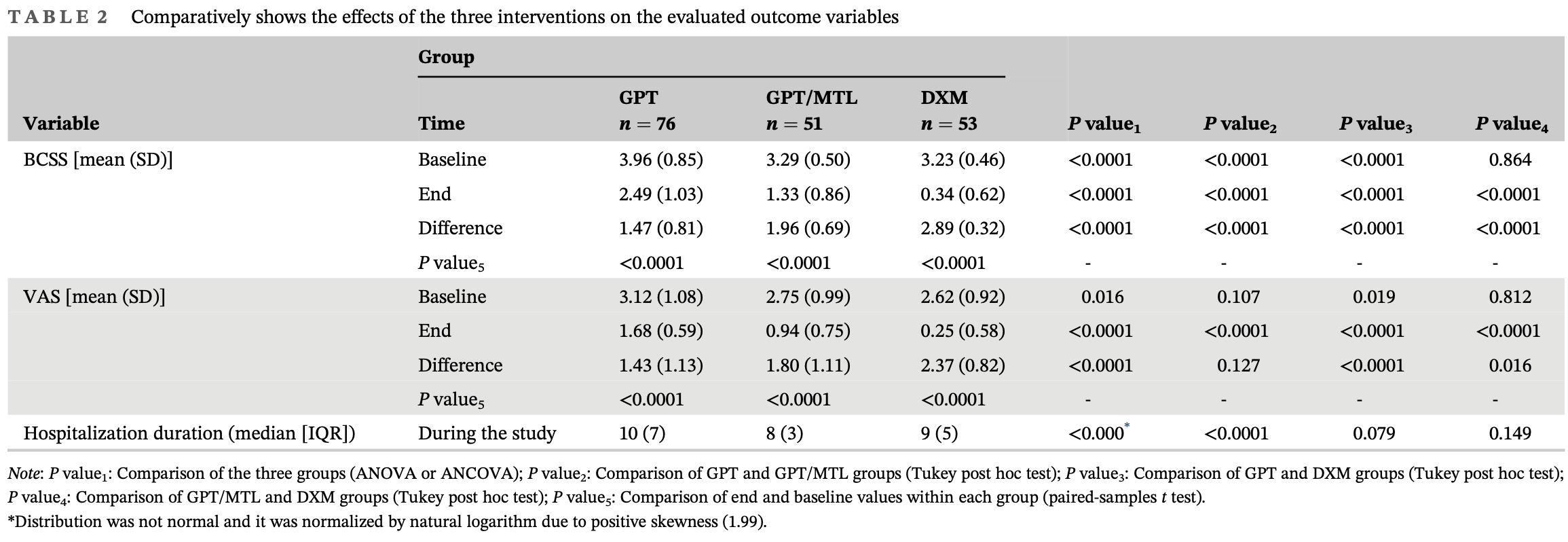
RCT 180 hospitalized COVID-19 patients showing improved cough symptoms and shorter hospitalization with montelukast/gabapentin compared to gabapentin. For gabapentin vs. dextromethorphan there was no significant difference in hospitalization and reduced improvement in cough symptoms.
Study covers montelukast and gabapentin.
|
hospitalization time, 20.0% lower, relative time 0.80, p = 0.01, treatment median 8.0 IQR 3.0 n=51, control median 10.0 IQR 7.0 n=76.
|
|
risk of no recovery, 25.0% lower, RR 0.75, p < 0.001, treatment mean 1.96 (±0.69) n=51, control mean 1.47 (±0.81) n=76, relative BCSS improvement, GPT/MTL vs. GPT.
|
|
risk of no recovery, 20.6% lower, RR 0.79, p = 0.07, treatment mean 1.8 (±1.11) n=51, control mean 1.43 (±1.13) n=76, relative VAS improvement, GPT/MTL vs. GPT.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Soltani et al., 31 Jul 2022, Randomized Controlled Trial, Iran, peer-reviewed, mean age 56.8, 6 authors, study period April 2020 - May 2020.
Contact: atousahakamifard@sbmu.ac.ir.
The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID‐19‐ related cough: A randomized, controlled clinical trial
The Clinical Respiratory Journal, doi:10.1111/crj.13529
Introduction: Cough is one of the most common presenting symptoms of COVID-19, which can persist for weeks or months. Objective: The goal of this study was to evaluate the effectiveness of gabapentin (GBT) alone and in combination with montelukast (MTL) for improving cough.
Methods: In this open-label randomized controlled clinical trial, eligible cases were patients hospitalized with moderate to severe COVID-19 who had cough with a Breathlessness, Cough, and Sputum Scale (BCSS) score of at least 2 based on its cough subscale. The participants were randomly assigned to three groups including two experimental groups and one control group. The first and second experimental groups received GBT and GBT/MTL, respectively, whereas the control group received dextromethorphan (DXM). Treatment duration was 5 days in all groups. Before and after the interventions, the severity of cough was evaluated using BCSS scale and Visual Analog Scale (VAS). Results: A total of 180 patients were included; GPT, GPT/MTL, and DXM consisted of 76, 51, and 53 patients, respectively. There was no significant difference between the three groups in terms of age, gender, and comorbidities (P > 0.05). Regarding BCSS and VAS scores, there was significant reduction from the baseline values in all groups (P < 0.0001), with the change rate being significantly higher in DXM group. The amount of reduction of BCSS in the GPT/MTL group was significantly more than the GPT group, whereas there was no significant difference between the two groups regarding VAS score. Although the duration of hospitalization differed between the groups with the GPT/MTL group having the shortest duration, the difference was statistically significant only between the GPT and GPT/MTL groups (P < 0.0001).
CONFLICT OF INTEREST The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS Rasool Soltani and Atousa Hakamifard designed the study, Sara Nasirharandi and Farzin Khorvash performed the study. Sara Nasirharandi collected the data. Maryam Nasirian analyzed the data. Rasool Soltani wrote the first draft of the manuscript. All authors have read and approved the final manuscript.
ETHICS STATEMENT The study was approved by the ethics committee of Isfahan University of Medical Sciences (ethics code: IR. MUI.MED.REC.1399.952).
References
Ahmed, Saleem, Naim, Soltani, Nasirharandi et al., The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID-19-related cough: A randomized, controlled clinical trial, Perspect Public Health, doi:info:doi/10.1111/crj.13529
Baldo, Opioid analgesic drugs and serotonin toxicity (syndrome): mechanisms, animal models, and links to clinical effects, Arch Toxicol, doi:info:doi/10.1007/s00204-018-2244-6
Canning, Chang, Bolser, Anatomy and neurophysiology of cough: CHEST guideline and expert panel report, Chest, doi:info:doi/10.1378/chest.14-1481
Chiu, Hehn, Woolf, Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology, Nat Neurosci, doi:info:doi/10.1038/nn.3144
Dong, Xu, Yu, Randomised clinical trial: gabapentin vs baclofen in the treatment of suspected refractory gastro-oesophageal reflux-induced chronic cough, Aliment Pharmacol Ther, doi:info:doi/10.1111/apt.15169
Gentile, Fireman, Skoner, Elevations of local leukotriene C4 levels during viral upper respiratory tract infections, Ann Allergy Asthma Immunol, doi:info:doi/10.1016/S1081-1206(10)63529-6
Gibson, Wang, Mcgarvey, Vertigan, Altman et al., Treatment of unexplained chronic cough: CHEST guideline and expert panel report, Chest, doi:info:doi/10.1378/chest.15-1496
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J med, doi:info:doi/10.1056/NEJMoa2002032
He, Deng, Li, Coronavirus disease 2019: what we know?, J med Virol, doi:info:doi/10.1002/jmv.25766
Kimos, Biggs, Mah, Analgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial, Pain, doi:info:doi/10.1016/j.pain.2006.08.028
Leidy, Schmier, Jones, Lloyd, Rocchiccioli, Evaluating symptoms in chronic obstructive pulmonary disease: validation of the Breathlessness, Cough and Sputum Scale, Respir med, doi:info:doi/10.1016/S0954-6111(03)80016-1
Matsuse, Hirose, Tsuchida, Effects of respiratory syncytial virus infection on dendritic cells and cysteinyl leukotrienes in lung tissues of a murine model of asthma, Allergol Int, doi:info:doi/10.2332/allergolint.O-06-476
Razzak, Waldfogel, Doberman, Feliciano, Smith, Gabapentin for cough in cancer, J Pain Palliat Care Pharmacother, doi:info:doi/10.1080/15360288.2017.1420120
Ryan, Birring, Gibson, Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial, Lancet, doi:info:doi/10.1016/S0140-6736(12)60776-4
Seymour, Gilby, Bardin, Rhinovirus infection increases 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects, J Infect Dis, doi:info:doi/10.1086/338570
Shah, Farrow, A commentary on "World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19), Int J Surg, doi:info:doi/10.1016/j.ijsu.2020.03.001
Stelmach, Korzeniewska, Stelmach, Majak, Grzelewski et al., Effects of montelukast treatment on clinical and inflammatory variables in patients with cystic fibrosis, Ann Allergy Asthma Immunol, doi:info:doi/10.1016/S1081-1206(10)61156-8
Trinh, Lee, Cao, Park, Asthma pharmacotherapy: an update on leukotriene treatments, Expert Rev Respir med, doi:info:doi/10.1080/17476348.2019.1670640
Verzele, Chua, Law, The impact of influenza pulmonary infection and inflammation on vagal bronchopulmonary sensory neurons, FASEB j, doi:info:doi/10.1096/fj.202001509R
Wang, Birring, Taylor, Montelukast for postinfectious cough in adults: a double-blind randomised placebocontrolled trial, Lancet Respir med, doi:info:doi/10.1016/S2213-2600(13)70245-5
DOI record:
{
"DOI": "10.1111/crj.13529",
"ISSN": [
"1752-6981",
"1752-699X"
],
"URL": "http://dx.doi.org/10.1111/crj.13529",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Introduction</jats:title><jats:p>Cough is one of the most common presenting symptoms of COVID‐19, which can persist for weeks or months.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>The goal of this study was to evaluate the effectiveness of gabapentin (GBT) alone and in combination with montelukast (MTL) for improving cough.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>In this open‐label randomized controlled clinical trial, eligible cases were patients hospitalized with moderate to severe COVID‐19 who had cough with a Breathlessness, Cough, and Sputum Scale (BCSS) score of at least 2 based on its cough subscale. The participants were randomly assigned to three groups including two experimental groups and one control group. The first and second experimental groups received GBT and GBT/MTL, respectively, whereas the control group received dextromethorphan (DXM). Treatment duration was 5 days in all groups. Before and after the interventions, the severity of cough was evaluated using BCSS scale and Visual Analog Scale (VAS).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A total of 180 patients were included; GPT, GPT/MTL, and DXM consisted of 76, 51, and 53 patients, respectively. There was no significant difference between the three groups in terms of age, gender, and comorbidities (<jats:italic>P</jats:italic> > 0.05). Regarding BCSS and VAS scores, there was significant reduction from the baseline values in all groups (<jats:italic>P</jats:italic> < 0.0001), with the change rate being significantly higher in DXM group. The amount of reduction of BCSS in the GPT/MTL group was significantly more than the GPT group, whereas there was no significant difference between the two groups regarding VAS score. Although the duration of hospitalization differed between the groups with the GPT/MTL group having the shortest duration, the difference was statistically significant only between the GPT and GPT/MTL groups (<jats:italic>P</jats:italic> < 0.0001).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>GPT, both alone and in combination with MTL, improves cough frequency and severity in hospitalized patients with COVID‐19, with the combination being more efficacious. This regimen may be useful in patients who cannot tolerate opioids.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/crj.13529"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-04-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-07-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-07-31"
}
],
"author": [
{
"affiliation": [
{
"name": "Infectious Diseases and Tropical Medicine Research Center Isfahan University of Medical Sciences Isfahan Iran"
},
{
"name": "Department of Clinical Pharmacy and Pharmacy Practice, School of Pharmacy and Pharmaceutical Sciences Isfahan University of Medical Sciences Isfahan Iran"
}
],
"family": "Soltani",
"given": "Rasool",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, School of Medicine Isfahan University of Medical Sciences Isfahan Iran"
}
],
"family": "Nasirharandi",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nosocomial Infection Research Center Isfahan University of Medical Sciences Isfahan Iran"
}
],
"family": "Khorvash",
"given": "Farzin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics and Epidemiology, School of Health Isfahan University of Medical Sciences Isfahan Iran"
}
],
"family": "Nasirian",
"given": "Maryam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases and Tropical Medicine Research Center Isfahan University of Medical Sciences Isfahan Iran"
}
],
"family": "Dolatshahi",
"given": "Kian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9456-2239",
"affiliation": [
{
"name": "Department of Infectious Diseases, School of Medicine Isfahan University of Medical Sciences Isfahan Iran"
},
{
"name": "Infectious Diseases and Tropical Medicine Research Center Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Hakamifard",
"given": "Atousa",
"sequence": "additional"
}
],
"container-title": "The Clinical Respiratory Journal",
"container-title-short": "Clinical Respiratory J",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T01:29:23Z",
"timestamp": 1659317363000
},
"deposited": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T15:15:16Z",
"timestamp": 1692717316000
},
"indexed": {
"date-parts": [
[
2024,
5,
22
]
],
"date-time": "2024-05-22T15:57:11Z",
"timestamp": 1716393431975
},
"is-referenced-by-count": 5,
"issue": "9",
"issued": {
"date-parts": [
[
2022,
7,
31
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2022,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
31
]
],
"date-time": "2022-07-31T00:00:00Z",
"timestamp": 1659225600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/crj.13529",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/crj.13529",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/crj.13529",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "604-610",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
7,
31
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
31
]
]
},
"published-print": {
"date-parts": [
[
2022,
9
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1002/jmv.25766",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1016/j.ijsu.2020.03.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"key": "e_1_2_10_4_1",
"unstructured": "https://covid19.who.int(Accessed: 29/1/2022)."
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1378/chest.14‐1481",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1096/fj.202001509R",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1038/nn.3144",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1378/chest.15‐1496",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1080/15360288.2017.1420120",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1086/338570",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1016/S1081‐1206(10)63529‐6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1080/17476348.2019.1670640",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1016/S0954‐6111(03)80016‐1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1016/S0140‐6736(12)60776‐4",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1111/apt.15169",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1016/j.pain.2006.08.028",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1016/S2213‐2600(13)70245‐5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1016/S1081‐1206(10)61156‐8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.2332/allergolint.O‐06‐476",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1007/s00204‐018‐2244‐6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1177/1757913914551914",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/crj.13529"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID‐19‐ related cough: A randomized, controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "16"
}
