Efficacy and safety of tixagevimab‐cilgavimab as pre‐exposure prophylaxis for COVID‐19: A systematic review and meta‐analysis
et al., Reviews in Medical Virology, doi:10.1002/rmv.2420, Jan 2023
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
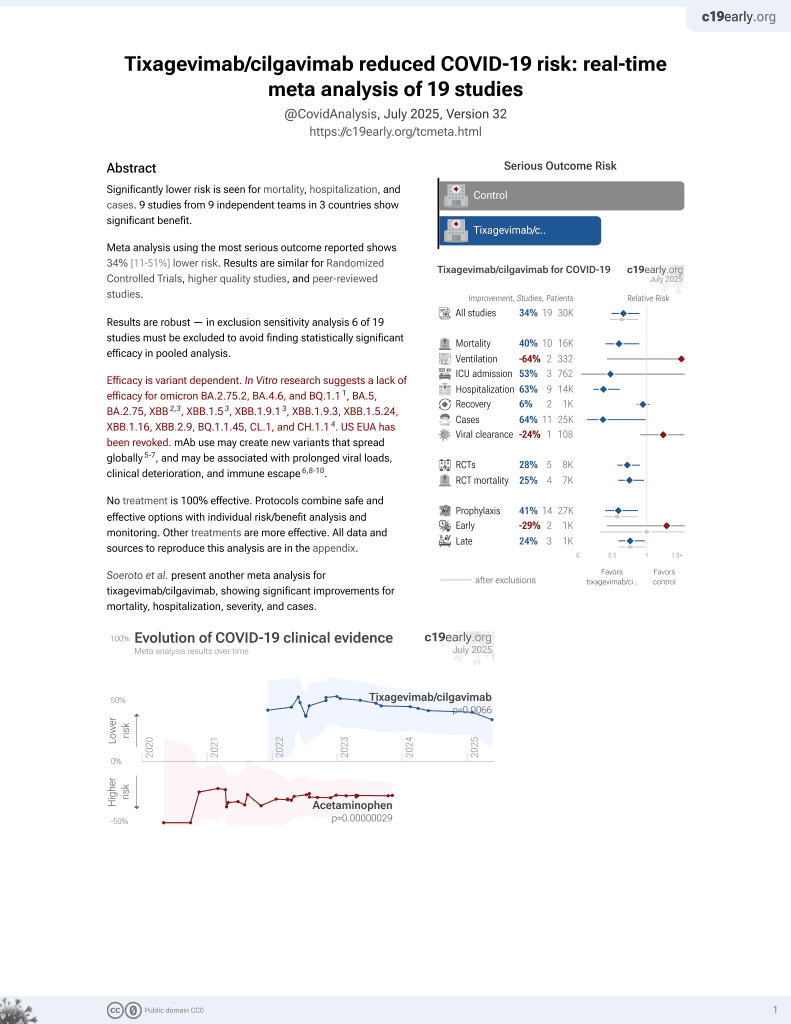
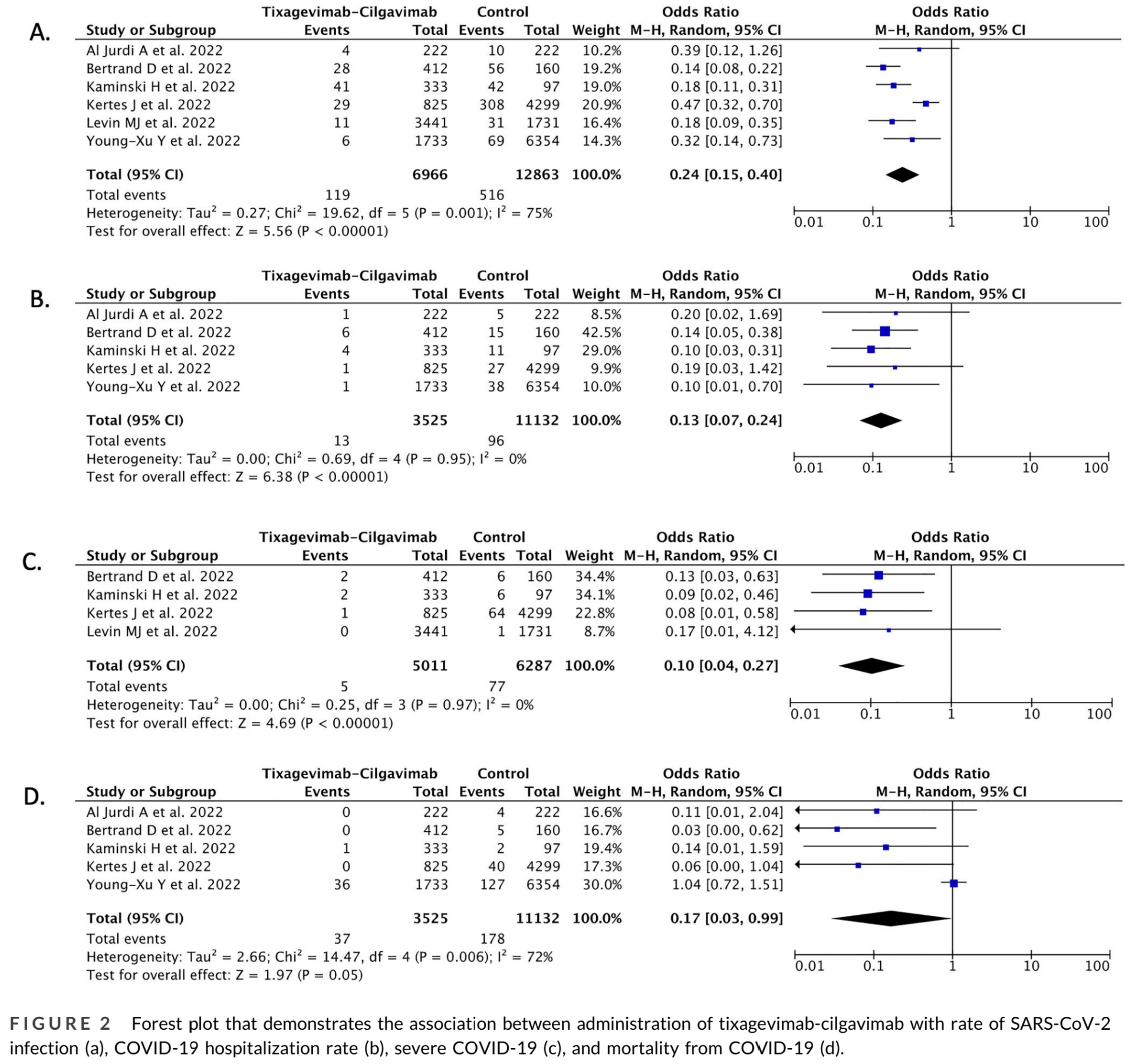
Meta analysis of 6 studies showing significantly lower COVID-19 cases, hospitalization, severity, and mortality with tixagevimab/cilgavimab prophylaxis.
Currently there are 19 tixagevimab/cilgavimab studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 40% lower [11‑60%] |
| Ventilation | 64% higher [-58‑541%] |
| ICU admission | 53% lower [-372‑95%] |
| Hospitalization | 63% lower [39‑77%] |
| Cases | 64% fewer [2‑87%] |
|
risk of death, 83.0% lower, OR 0.17, p = 0.047, RR approximated with OR.
|
|
risk of hospitalization, 87.0% lower, OR 0.13, p < 0.001, RR approximated with OR.
|
|
risk of severe case, 87.0% lower, OR 0.13, p < 0.001, RR approximated with OR.
|
|
risk of case, 76.0% lower, OR 0.24, p < 0.001, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Soeroto et al., 8 Jan 2023, peer-reviewed, 4 authors.
DOI record:
{
"DOI": "10.1002/rmv.2420",
"ISSN": [
"1052-9276",
"1099-1654"
],
"URL": "http://dx.doi.org/10.1002/rmv.2420",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Some proportions of populations, such as immunocompromised patients and organ transplant recipients might have inadequate immune responses to the vaccine for coronavirus disease 2019 (COVID‐19). For these groups of populations, administering monoclonal antibodies might offer some additional protection. This review sought to analyze the effectiveness and safety of tixagevimab‐cilgavimab (Evusheld) as pre‐exposure prophylaxis against COVID‐19. We used specific keywords to comprehensively search for potential studies on PubMed, Scopus, Europe PMC, and <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" xlink:href=\"http://ClinicalTrials.gov\">ClinicalTrials.gov</jats:ext-link> sources until 3 September 2022. We collected all published articles that analyzed tixagevimab‐cilgavimab on the course of COVID‐19. Review Manager 5.4 was utilized for statistical analysis. Six studies were included. Our pooled analysis revealed that tixagevimab‐cilgavimab prophylaxis may decrease the rate of SARS‐CoV‐2 infection (OR: 0.24; 95% CI: 0.15–0.40, <jats:italic>p</jats:italic> < 0.00001, <jats:italic>I</jats:italic><jats:sup><jats:italic>2</jats:italic></jats:sup> = 75%), lower COVID‐19 hospitalization rate (OR: 0.13; 95% CI: 0.07–0.24, <jats:italic>p</jats:italic> < 0.00001, <jats:italic>I</jats:italic><jats:sup>2</jats:sup> = 0%), decrease the severity risk (OR: 0.13; 95% CI: 0.07–0.24, <jats:italic>p</jats:italic> < 0.00001, <jats:italic>I</jats:italic><jats:sup>2</jats:sup> = 0%), and lower COVID‐19 deaths (OR: 0.17; 95% CI: 0.03–0.99, <jats:italic>p</jats:italic> = 0.05, <jats:italic>I</jats:italic><jats:sup>2</jats:sup> = 72%). In the included studies, no major adverse events were reported. This study proposes that tixagevimab‐cilgavimab was effective and safe for preventing COVID‐19. Tixagevimab‐cilgavimab may be offered to those who cannot be vaccinated or have inadequate immune response from the COVID‐19 vaccine to give additional protection.</jats:p>",
"alternative-id": [
"10.1002/rmv.2420"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-11-15"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-12-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-01-08"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Internal Medicine Division of Pulmonology and Critical Illness Padjadjaran University Bandung West Java Indonesia"
}
],
"family": "Soeroto",
"given": "Arto Yuwono",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Faculty of Medicine Pelita Harapan University Tangerang Indonesia"
}
],
"family": "Yanto",
"given": "Theo Audi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5219-9029",
"affiliation": [
{
"name": "Department of Internal Medicine Faculty of Medicine Pelita Harapan University Tangerang Indonesia"
}
],
"authenticated-orcid": false,
"family": "Kurniawan",
"given": "Andree",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1748-9776",
"affiliation": [
{
"name": "Faculty of Medicine Pelita Harapan University Tangerang Indonesia"
}
],
"authenticated-orcid": false,
"family": "Hariyanto",
"given": "Timotius Ivan",
"sequence": "additional"
}
],
"container-title": "Reviews in Medical Virology",
"container-title-short": "Reviews in Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
1,
9
]
],
"date-time": "2023-01-09T05:38:56Z",
"timestamp": 1673242736000
},
"deposited": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T05:35:55Z",
"timestamp": 1711690555000
},
"indexed": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T05:06:19Z",
"timestamp": 1722488779796
},
"is-referenced-by-count": 22,
"issue": "2",
"issued": {
"date-parts": [
[
2023,
1,
8
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2023,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
8
]
],
"date-time": "2023-01-08T00:00:00Z",
"timestamp": 1673136000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/rmv.2420",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/rmv.2420",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/rmv.2420",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
1,
8
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
8
]
]
},
"published-print": {
"date-parts": [
[
2023,
3
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.jpsychires.2021.08.031",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"key": "e_1_2_10_3_1",
"unstructured": "World Health Organization.Coronavirus Disease (COVID‐19): Situation Report;2022. Accessed September 1.https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐COVID‐19–‐31‐august‐2022"
},
{
"DOI": "10.1016/j.archger.2020.104299",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.1038/s41440‐020‐0515‐0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1016/j.diabres.2021.109031",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1016/j.diabres.2022.110205",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1016/j.yebeh.2021.108437",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/j.ijcha.2020.100557",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1056/NEJMcp2009575",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.15585/mmwr.mm7030e2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1016/j.biopha.2021.111272",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.52225/narra.v2i3.92",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.52225/narra.v2i3.88",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1002/jmv.27730",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1002/jmv.27623",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1186/s13054‐020‐03400‐9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1002/jmv.26698",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1080/14787210.2021.1982695",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1056/NEJMoa2116620",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1111/ajt.17121",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1093/ofid/ofac283",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1016/j.ccell.2022.05.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1001/jamasurg.2021.0546",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1136/bmj.n71",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"key": "e_1_2_10_26_1",
"unstructured": "National Health Commission of the People’s Republic of China.Diagnosis and Treatment of New Coronavirus Pneumonitis. (Trial Version 5).http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.2147/CLEP.S66677",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1111/ajt.17128",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1016/j.kint.2022.05.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_1"
},
{
"DOI": "10.1016/j.kint.2022.07.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"DOI": "10.1093/cid/ciac625",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"DOI": "10.1101/2022.05.28.22275716",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_1"
},
{
"key": "e_1_2_10_34_1",
"unstructured": "World Health Organization. Tracking SARS‐CoV‐2 variant. Accessed December 14 2022.https://www.who.int/activities/tracking‐SARS‐CoV‐2‐variants"
},
{
"key": "e_1_2_10_35_1",
"unstructured": "GÉODES.Géo Données En Santé Publique;2022. Accessed December 14.https://geodes.santepubliquefrance.fr"
},
{
"DOI": "10.1056/NEJMoa2201570",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_36_1"
},
{
"key": "e_1_2_10_37_1",
"unstructured": "Centers for Disease Control and Prevention.First Confirmed Case of Omicron Variant Detected in the United States;2022. Accessed December 14.https://www.cdc.gov/media/releases/2021/s1201‐omicron‐variant.html"
},
{
"DOI": "10.1016/s0895‐4356(99)00161‐4",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_1"
},
{
"DOI": "10.1002/sim.1461",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_39_1"
},
{
"key": "e_1_2_10_40_1",
"unstructured": "United States Food and Drug Administration (FDA).FDA limits use of certain monoclonal antibodies to treat COVID‐19 due to the omicron variant.2022. Accessed December 14.https://ldh.la.gov/assets/oph/Center‐CP/HANs/2022/HANS22‐07‐FDALimitsUse‐Certain‐Monoclonal‐Antibodies‐Omicron‐Variant.pdf"
},
{
"DOI": "10.1056/NEJMc2119407",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_41_1"
},
{
"DOI": "10.1128/spectrum.00926‐22",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_42_1"
},
{
"DOI": "10.1016/j.amjmed.2022.06.019",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_43_1"
},
{
"DOI": "10.1016/j.jiph.2022.11.024",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_44_1"
},
{
"DOI": "10.1007/s40265‐022‐01731‐1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_45_1"
},
{
"DOI": "10.1126/scitranslmed.abl8124",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_46_1"
},
{
"DOI": "10.1371/journal.pmed.1003917",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_47_1"
},
{
"DOI": "10.1001/jama.2022.13214",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_48_1"
},
{
"DOI": "10.1016/j.antiviral.2020.104820",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_49_1"
},
{
"DOI": "10.1038/s41591‐022‐01792‐5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_50_1"
},
{
"DOI": "10.3390/diagnostics1106109",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_51_1"
},
{
"DOI": "10.4269/ajtmh.20‐1110",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_52_1"
}
],
"reference-count": 51,
"references-count": 51,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/rmv.2420"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of tixagevimab‐cilgavimab as pre‐exposure prophylaxis for COVID‐19: A systematic review and meta‐analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "33"
}