Early Use of Ibuprofen in Moderate Cases of COVID-19 Might be a Promising Agent to Attenuate the Severity of Disease: A Randomized Controlled Trial
et al., The Open Anesthesia Journal, doi:10.2174/25896458-v17-e230403-2022-26, PACTR202202880140319, Apr 2023
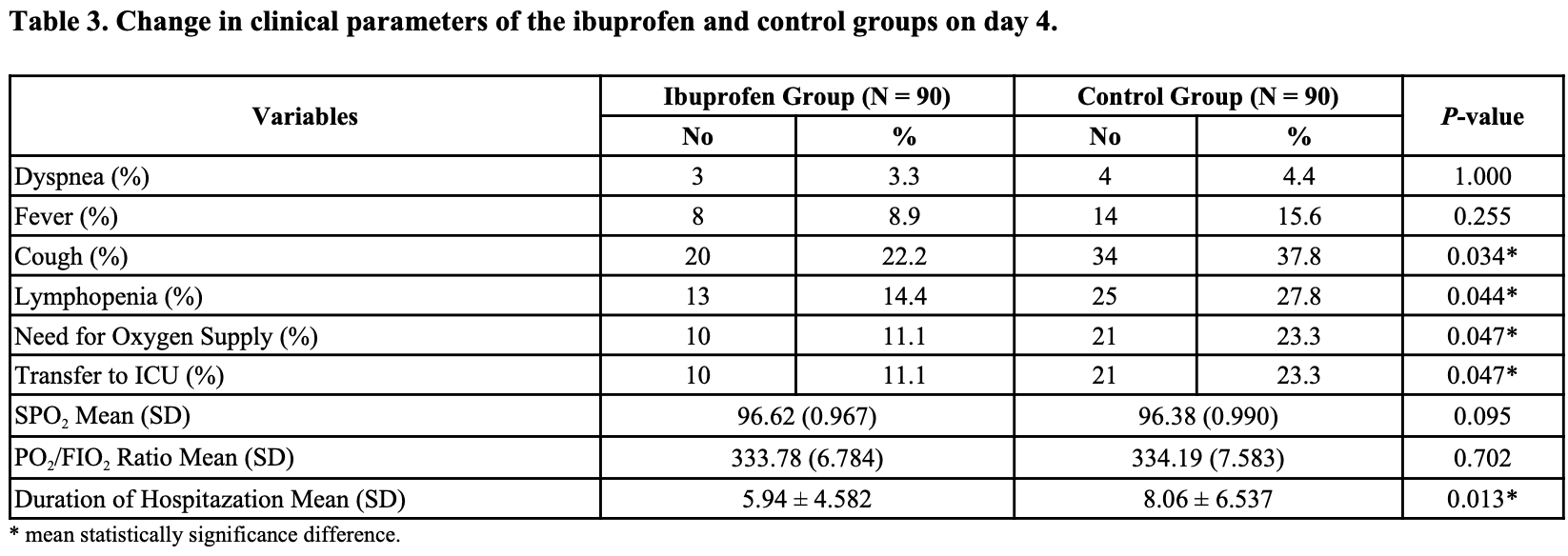
RCT 180 moderate hospitalized COVID-19 patients in Egypt, showing lower ICU admission and shorter hospitalization with ibuprofen compared with acetaminophen.
Study covers acetaminophen and ibuprofen.
|
risk of ICU admission, 52.4% lower, RR 0.48, p = 0.047, treatment 10 of 90 (11.1%), control 21 of 90 (23.3%), NNT 8.2.
|
|
risk of oxygen therapy, 52.4% lower, RR 0.48, p = 0.047, treatment 10 of 90 (11.1%), control 21 of 90 (23.3%), NNT 8.2.
|
|
hospitalization time, 26.3% lower, relative time 0.74, p = 0.01, treatment 90, control 90.
|
|
risk of no recovery, 25.0% lower, RR 0.75, p = 1.00, treatment 3 of 90 (3.3%), control 4 of 90 (4.4%), NNT 90, day 4, dyspnea.
|
|
risk of no recovery, 42.9% lower, RR 0.57, p = 0.25, treatment 8 of 90 (8.9%), control 14 of 90 (15.6%), NNT 15, day 4, fever.
|
|
risk of no recovery, 48.0% lower, RR 0.52, p = 0.04, treatment 13 of 90 (14.4%), control 25 of 90 (27.8%), NNT 7.5, day 4, lymphopenia.
|
|
risk of no recovery, 41.2% lower, RR 0.59, p = 0.03, treatment 20 of 90 (22.2%), control 34 of 90 (37.8%), NNT 6.4, day 4, cough.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sobhy et al., 19 Apr 2023, Double Blind Randomized Controlled Trial, Egypt, peer-reviewed, 6 authors, study period January 2022 - May 2022, this trial compares with another treatment - results may be better when compared to placebo, trial PACTR202202880140319.
Contact: dr.amrsobhy2013@gmail.com.
Early Use of Ibuprofen in Moderate Cases of COVID-19 Might be a Promising Agent to Attenuate the Severity of Disease: A Randomized Controlled Trial
The Open Anesthesia Journal, doi:10.2174/25896458-v17-e230403-2022-26
Introduction: Critically ill COVID-19 patients undergoing cytokine storm are believed to have a worse prognosis and increased fatality rate. Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAIDs) that might prove beneficial for the early management of COVID-19 due to its immunomodulatory effects. This study aimed to assess the efficacy and safety of the early use of ibuprofen to attenuate the severity of the course of COVID-19 and improve outcomes in patients diagnosed with a moderate case of COVID-19 disease.
Methods: This randomized, double-blinded prospective study was conducted from January, 2022 to May, 2022, which included a total sample size of 180 patients with moderate cases of COVID-19. The number of patients transferred to intensive care was used as a primary outcome with a proposed large effect size (0.8), alfa =0.05, and power=0.80, so 90 cases were included in each group. Secondary outcomes were inflammatory markers: C-Reactive Protein (CRP), serum ferritin, and interleukin-6 (IL-6), duration of hospital stay, and need for ICU admission.
Results: One hundred eighty patients with moderate case of COVID-19 disease were divided in a 1: 1 ratio to receive ibuprofen (IG) or paracetamol (CG). The average age of the included patients was almost 41 years. Statistically significant differences were reported between both groups in terms of improvement in cough symptoms and lymphopenia in IG compared to CG (p= 0.034 and p= 0.044, respectively). Regarding secondary outcomes, statistically, significant differences were reported between the study's groups in terms of the mean number of patients transferred to the ICU in IG compared to the CG (p =0.0.047) and duration of hospitalization (p =0.013), with no significant differences (p > 0.9999) in the occurrence of side effects. Concerning the follow-up of the cytokine storm marker, there was a statistically significant reduction in serum cytokine marker compared to the baseline value (P < 0.05) in the same group. No statistically significant differences were observed when comparing both groups together in terms of serum ferritin level (p =0.570), serum IL-6 level (p =0.580), and CRP level (p =0.401).
Conclusion: It can be concluded that early use of ibuprofen as adjuvant therapy in COVID-19 management is effective and safe to attenuate the severity of diseases and improve outcomes.
STANDARDS OF REPORTING CONSORT guidelines and methodology were followed.
CONFLICT OF INTEREST The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIALS Supplementary material is available on the Publisher's website.
References
Bhaskar, Sinha, Banach, Mittoo, Weissert et al., None
Fisman, Shapira, Motro, Pines, Tenenbaum et al., None
Hashimoto, Graham, Geraci, Signaling through the [10] prostaglandin I2 receptor IP protects against respiratory syncytial virus-induced illness, J Virol
Kelleni, Early use of non-steroidal anti-inflammatory drugs in
Kelleni, Nitazoxanide/azithromycin combination for
Lu, Zhang, Zhan, Preventing mortality in COVID-19
Mirzaei, Karampoor, Sholeh, Moradi, Ranjbar et al., None
Qin, Zhou, Hu, Dysregulation of immune response in
Ramaswamy, Bhargava, Panda, Ostwal, Repurposing, None, Clin Infect Dis, doi:10.1093/cid/ciaa248
Thomas, Non-steroidal anti-inflammatory drug use and outcomes of
DOI record:
{
"DOI": "10.2174/25896458-v17-e230403-2022-26",
"ISSN": [
"2589-6458"
],
"URL": "http://dx.doi.org/10.2174/25896458-v17-e230403-2022-26",
"abstract": "<jats:sec>\n <jats:title>Introduction:</jats:title>\n <jats:p>Critically ill COVID-19 patients undergoing cytokine storm are believed to have a worse prognosis and increased fatality rate. Ibuprofen is a non-steroidal anti-inflammatory drug (NSAIDs) that might prove beneficial for the early management of COVID-19 due to its immunomodulatory effects. This study aimed to assess the efficacy and safety of the early use of ibuprofen to attenuate the severity of the course of COVID-19 and improve outcomes in patients diagnosed with a moderate case of COVID-19 disease.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods:</jats:title>\n <jats:p>This randomized, double-blinded prospective study was conducted from January, 2022 to May, 2022, which included a total sample size of 180 patients with moderate cases of COVID-19. The number of patients transferred to intensive care was used as a primary outcome with a proposed large effect size (0.8), alfa =0.05, and power=0.80, so 90 cases were included in each group. Secondary outcomes were inflammatory markers: C-Reactive Protein (CRP), serum ferritin, and interleukin-6 (IL-6), duration of hospital stay, and need for ICU admission.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results:</jats:title>\n <jats:p>One hundred eighty patients with moderate case of COVID-19 disease were divided in a 1: 1 ratio to receive ibuprofen (IG) or paracetamol (CG). The average age of the included patients was almost 41 years. Statistically significant differences were reported between both groups in terms of improvement in cough symptoms and lymphopenia in IG compared to CG (<jats:italic>p</jats:italic>= 0.034 and <jats:italic>p</jats:italic>= 0.044, respectively). Regarding secondary outcomes, statistically, significant differences were reported between the study’s groups in terms of the mean number of patients transferred to the ICU in IG compared to the CG (<jats:italic>p</jats:italic> =0.0.047) and duration of hospitalization (<jats:italic>p</jats:italic> =0.013), with no significant differences (<jats:italic>p</jats:italic> > 0.9999) in the occurrence of side effects.</jats:p>\n <jats:p>Concerning the follow-up of the cytokine storm marker, there was a statistically significant reduction in serum cytokine marker compared to the baseline value (<jats:italic>P</jats:italic> < 0.05) in the same group. No statistically significant differences were observed when comparing both groups together in terms of serum ferritin level (<jats:italic>p</jats:italic> =0.570), serum IL-6 level (<jats:italic>p</jats:italic> =0.580), and CRP level (<jats:italic>p</jats:italic> =0.401).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion:</jats:title>\n <jats:p>It can be concluded that early use of ibuprofen as adjuvant therapy in COVID-19 management is effective and safe to attenuate the severity of diseases and improve outcomes.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Trial Registration:</jats:title>\n <jats:p>Project manager for the Pan African Clinical Trial Registry PACTR202202880140319. Registered 9<jats:sup>th</jats:sup> February, 2022 - Retrospectively registered, (https://pactr.samrc.ac.za/)</jats:p>\n </jats:sec>",
"assertion": [
{
"group": {
"label": "Peer Review Details",
"name": "peer_review_details"
},
"label": "Review Status",
"name": "review_status",
"order": 0,
"value": "Peer Reviewed"
},
{
"group": {
"label": "Peer Review Details",
"name": "peer_review_details"
},
"label": "Review Process",
"name": "review_process",
"order": 1,
"value": "Single blind"
},
{
"group": {
"label": "Plagiarism Screening",
"name": "plagiarism_screening"
},
"label": "Screening Status",
"name": "screening_status",
"order": 0,
"value": "Checked with iThenticate"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-10-24"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2023-1-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2023-1-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2023-04-19"
}
],
"author": [
{
"affiliation": [],
"family": "Sobhy",
"given": "Amr",
"sequence": "first"
},
{
"affiliation": [],
"family": "Saleh",
"given": "Lobna A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AbdelAtty",
"given": "Marwa E.l",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AbdelAtty",
"given": "Marwa E.l",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Refaat",
"given": "Sameh A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamal",
"given": "Mohammed",
"sequence": "additional"
}
],
"container-title": "The Open Anesthesia Journal",
"container-title-short": "TOATJ",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"openanesthesiajournal.com",
"benthamopen.com"
]
},
"created": {
"date-parts": [
[
2023,
4,
20
]
],
"date-time": "2023-04-20T04:07:33Z",
"timestamp": 1681963653000
},
"deposited": {
"date-parts": [
[
2023,
4,
20
]
],
"date-time": "2023-04-20T04:07:39Z",
"timestamp": 1681963659000
},
"indexed": {
"date-parts": [
[
2023,
4,
21
]
],
"date-time": "2023-04-21T06:39:58Z",
"timestamp": 1682059198109
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
4,
19
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023,
4,
19
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/legalcode",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
19
]
],
"date-time": "2023-04-19T00:00:00Z",
"timestamp": 1681862400000
}
}
],
"link": [
{
"URL": "https://openanesthesiajournal.com/contents/volumes/V17/e258964582303020/e258964582303020.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://openanesthesiajournal.com/contents/volumes/V17/e258964582303020/e258964582303020.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://openanesthesiajournal.com/contents/volumes/V17/e258964582303020/e258964582303020.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "965",
"original-title": [],
"prefix": "10.2174",
"published": {
"date-parts": [
[
2023,
4,
19
]
]
},
"published-print": {
"date-parts": [
[
2023,
4,
19
]
]
},
"publisher": "Bentham Science Publishers Ltd.",
"reference": [
{
"DOI": "10.1007/s11033-020-05621-1",
"doi-asserted-by": "publisher",
"key": "ref1",
"unstructured": "Mirzaei R, Karampoor S, Sholeh M, Moradi P, Ranjbar R, Ghasemi F. \n A contemporary review on pathogenesis and immunity of COVID-19 infection. \n Mol Biol Rep \n 2020; \n 47\n (7)\n : 5365-76."
},
{
"DOI": "10.3389/fimmu.2020.01648",
"doi-asserted-by": "crossref",
"key": "ref2",
"unstructured": "Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS. \n Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: The REPROGRAM Consortium Position Paper. \n Front Immunol \n 2020; \n 11\n : 1648."
},
{
"DOI": "10.1016/j.biopha.2020.110982",
"doi-asserted-by": "publisher",
"key": "ref3",
"unstructured": "Kelleni MT. \n Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. \n Biomed Pharmacother \n 2021; \n 133\n : 110982."
},
{
"key": "ref4",
"unstructured": "Available from: https://hiph.alexu.edu.eg/images/egyptian_national_guidelines_covid-19.pdf"
},
{
"key": "ref5",
"unstructured": "Fisman EZ, Shapira I, Motro M, Pines A, Tenenbaum A. \n The combined cough frequency/severity scoring: A new approach to cough evaluation in clinical settings. \n J Med \n 2001; \n 32\n (3-4)\n : 181-7."
},
{
"DOI": "10.3389/fcell.2020.00677",
"doi-asserted-by": "publisher",
"key": "ref6",
"unstructured": "Lu L, Zhang H, Zhan M, et al. \n Preventing mortality in COVID-19 patients: Which cytokine to target in a raging storm? \n Front Cell Dev Biol \n 2020; \n 8\n : 677."
},
{
"DOI": "10.1016/j.phrs.2020.104874",
"doi-asserted-by": "publisher",
"key": "ref7",
"unstructured": "Kelleni MT. \n Nitazoxanide/azithromycin combination for COVID-19: A suggested new protocol for early management. \n Pharmacol Res \n 2020; \n 157\n (July)\n : 104874."
},
{
"DOI": "10.1093/cid/ciaa248",
"doi-asserted-by": "publisher",
"key": "ref8",
"unstructured": "Qin C, Zhou L, Hu Z, et al. \n Dysregulation of immune response in patients with COVID-19 in Wuhan, China. \n Clin Infect Dis \n 2020; \n 71\n (15)\n : 762-8."
},
{
"DOI": "10.4103/CRST.CRST_156_20",
"doi-asserted-by": "publisher",
"key": "ref9",
"unstructured": "Ramaswamy A, Bhargava P, Panda P, Ostwal V. \n Repurposing valproate to prevent acute respiratory distress syndrome/acute lung injury in COVID-19: A review of immunomodulatory action. \n Cancer Research, Statistics, and Treatment \n 2020; \n 3\n (5)\n : 65."
},
{
"DOI": "10.1128/JVI.78.19.10303-10309.2004",
"doi-asserted-by": "crossref",
"key": "ref10",
"unstructured": "Hashimoto K, Graham BS, Geraci MW, et al. \n Signaling through the prostaglandin I2 receptor IP protects against respiratory syncytial virus-induced illness. \n J Virol \n 2004; \n 78\n (19)\n : 10303-9."
},
{
"DOI": "10.1016/S2665-9913(21)00104-1",
"doi-asserted-by": "publisher",
"key": "ref11",
"unstructured": "Thomas M. \n Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: A matched, prospective cohort study. \n Lancet Rheumatol \n 2021; \n 3\n (7)\n : 498-506."
}
],
"reference-count": 11,
"references-count": 11,
"relation": {},
"resource": {
"primary": {
"URL": "https://openanesthesiajournal.com/VOLUME/17/ELOCATOR/e258964582303020/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Anesthesiology and Pain Medicine"
],
"subtitle": [],
"title": "Early Use of Ibuprofen in Moderate Cases of COVID-19 Might be a Promising Agent to Attenuate the Severity of Disease: A Randomized Controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.2174/crossmark_policy",
"volume": "17"
}