The Impact of Androgen Deprivation Therapy on COVID-19 Illness in Men With Prostate Cancer
et al., JNCI Cancer Spectrum, doi:10.1093/jncics/pkac035, May 2022
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
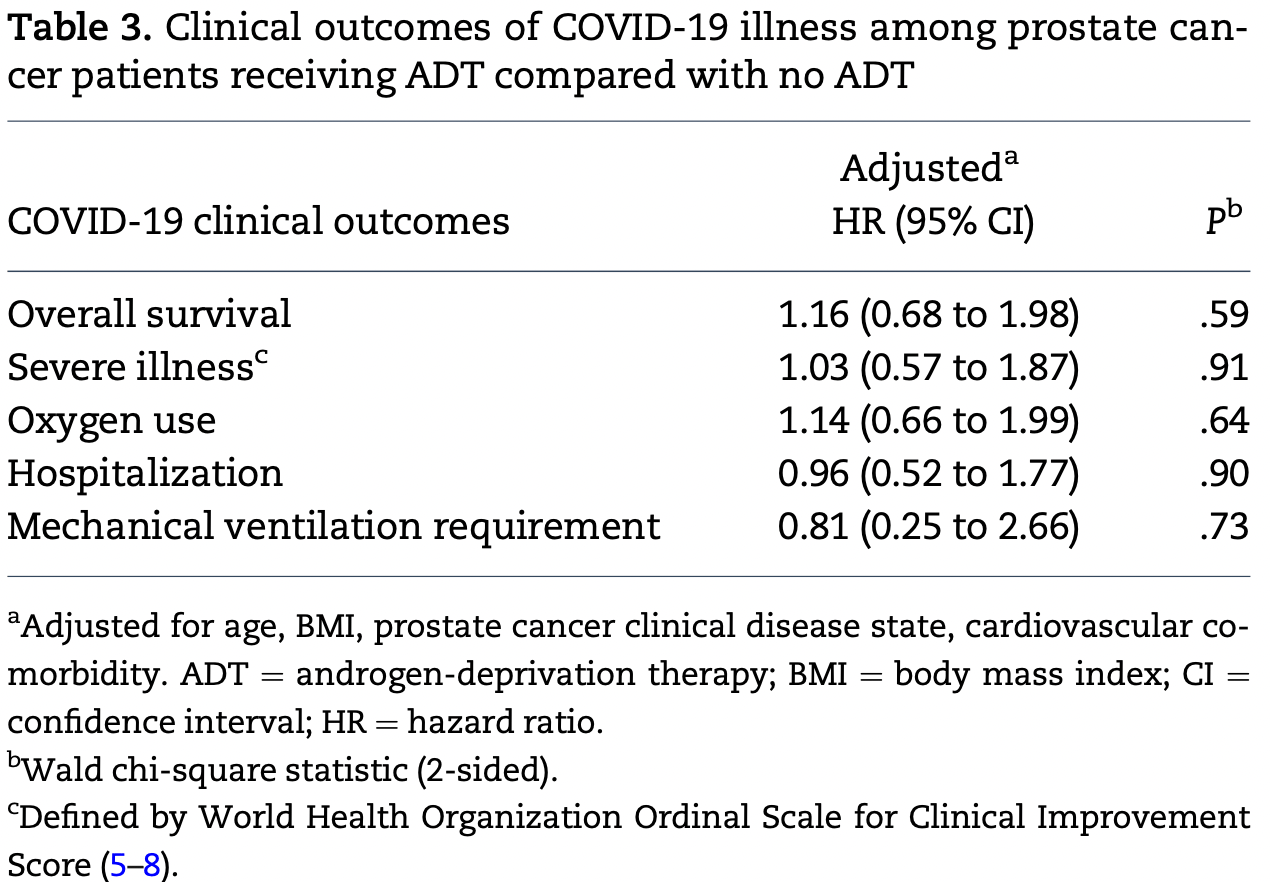
Retrospective 465 prostate cancer patients, showing no significant difference in COVID-19 outcomes with ADT.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 16.0% higher, HR 1.16, p = 0.59, treatment 148, control 317.
|
|
risk of mechanical ventilation, 19.0% lower, HR 0.81, p = 0.73, treatment 148, control 317.
|
|
risk of severe case, 3.0% higher, HR 1.03, p = 0.91, treatment 148, control 317.
|
|
risk of hospitalization, 4.0% lower, HR 0.96, p = 0.90, treatment 148, control 317.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shah et al., 12 May 2022, retrospective, USA, peer-reviewed, median age 71.0, 22 authors, study period 1 March, 2020 - 31 May, 2020.
Contact: william.oh@mssm.edu.
The Impact of Androgen Deprivation Therapy on COVID-19 Illness in Men With Prostate Cancer
JNCI Cancer Spectrum, doi:10.1093/jncics/pkac035
Background: TMPRSS2, a cell surface protease regulated by androgens and commonly upregulated in prostate cancer (PCa), is a necessary component for SARS-CoV-2 viral entry into respiratory epithelial cells. Previous reports suggested a lower risk of SARS-CoV-2 among PCa patients on androgen deprivation therapy (ADT). However, the impact of ADT on severe COVID-19 illness is poorly understood. Methods: We performed a multicenter study across 7 US medical centers and evaluated patients with PCa and SARS-CoV-2 detected by polymerase-chain-reaction between March 1, 2020, and May 31, 2020. PCa patients were considered on ADT if they had received appropriate ADT treatment within 6 months of COVID-19 diagnosis. We used multivariable logistic and Cox proportional-hazard regression models for analysis. All statistical tests were 2-sided. Results: We identified 465 PCa patients (median age ¼ 71 years) with a median follow-up of 60 days. Age, body mass index, cardiovascular comorbidity, and PCa clinical disease state adjusted overall survival (hazard ratio [HR] ¼ 1.16, 95% confidence interval [CI] ¼ 0.68 to 1.98, P ¼ .59), hospitalization status (HR ¼ 0.96, 95% CI ¼ 0.52 to 1.77, P ¼ .90), supplemental oxygenation (HR 1.14, 95% CI ¼ 0.66 to 1.99, P ¼ .64), and use of mechanical ventilation (HR ¼ 0.81, 95% CI ¼ 0.25 to 2.66, P ¼ .73) were similar between ADT and non-ADT cohorts. Similarly, the addition of androgen receptor-directed therapy within 30 days of COVID-19 diagnosis to ADT vs ADT alone did not statistically significantly affect overall survival (androgen receptor-directed therapy: HR ¼ 1.27, 95% CI ¼ 0.69 to 2.32, P ¼ .44). Conclusions: In this retrospective cohort of PCa patients, the use of ADT was not demonstrated to influence severe COVID-19 outcomes, as defined by hospitalization, supplemental oxygen use, or death. Age 70 years and older was statistically significantly associated with a higher risk of developing severe COVID-19 disease.
Notes Role of the funder: The design, interpretation, and analysis of this study, the writing of the manuscript, and decision to submit the manuscript for publication rest solely with the authors.
References
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19-final report, N Engl J Med
Caffo, Zagonel, Baldessari, On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2, Ann Oncol
Chaipan, Kobasa, Bertram, Proteolytic activation of the 1918 influvirus hemagglutinin, J Virol
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Iwata-Yoshikawa, Okamura, Shimizu, Hasegawa, Takeda et al., TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection, J Virol
Klein, Li, Milinovich, Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2, J Urol
Koskinen, Carpen, Honkanen, Androgen deprivation and SARS-CoV-2 in men with prostate cancer, Ann Oncol
Kwon, Vashisht, Borno, Androgen-deprivation therapy and SARS-CoV-2 in men with prostate cancer: findings from the University of California Health System registry, Ann Oncol
Li, Han, Dai, Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide, Nat Commun
Lin, Ferguson, White, Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2, Cancer Res
Matsuyama, Nagata, Shirato, Kawase, Takeda et al., Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2, J Virol
Mikkonen, Pihlajamaa, Sahu, Zhang, Janne, Androgen receptor and androgen-dependent gene expression in lung, Mol Cell Endocrinol
Montopoli, Zumerle, Vettor, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n¼4532), Ann. Oncol
Mostaghel, Nelson, Lange, Targeted androgen pathway suppression in localized prostate cancer: a pilot study, J Clin Oncol
Patel, Zhong, Liaw, Does androgen deprivation therapy protect against severe complications from COVID-19?, Ann Oncol
Richardson, Hirsch, Narasimhan, the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA
Schmidt, Tucker, Bakouny, Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19, JAMA Netw Open
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA
DOI record:
{
"DOI": "10.1093/jncics/pkac035",
"ISSN": [
"2515-5091"
],
"URL": "http://dx.doi.org/10.1093/jncics/pkac035",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>TMPRSS2, a cell surface protease regulated by androgens and commonly upregulated in prostate cancer (PCa), is a necessary component for SARS-CoV-2 viral entry into respiratory epithelial cells. Previous reports suggested a lower risk of SARS-CoV-2 among PCa patients on androgen deprivation therapy (ADT). However, the impact of ADT on severe COVID-19 illness is poorly understood.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a multicenter study across 7 US medical centers and evaluated patients with PCa and SARS-CoV-2 detected by polymerase-chain-reaction between March 1, 2020, and May 31, 2020. PCa patients were considered on ADT if they had received appropriate ADT treatment within 6 months of COVID-19 diagnosis. We used multivariable logistic and Cox proportional-hazard regression models for analysis. All statistical tests were 2-sided.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>We identified 465 PCa patients (median age = 71 years) with a median follow-up of 60 days. Age, body mass index, cardiovascular comorbidity, and PCa clinical disease state adjusted overall survival (hazard ratio [HR] = 1.16, 95% confidence interval [CI] = 0.68 to 1.98, P = .59), hospitalization status (HR = 0.96, 95% CI = 0.52 to 1.77, P = .90), supplemental oxygenation (HR 1.14, 95% CI = 0.66 to 1.99, P = .64), and use of mechanical ventilation (HR = 0.81, 95% CI = 0.25 to 2.66, P = .73) were similar between ADT and non-ADT cohorts. Similarly, the addition of androgen receptor–directed therapy within 30 days of COVID-19 diagnosis to ADT vs ADT alone did not statistically significantly affect overall survival (androgen receptor–directed therapy: HR = 1.27, 95% CI = 0.69 to 2.32, P = .44).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>In this retrospective cohort of PCa patients, the use of ADT was not demonstrated to influence severe COVID-19 outcomes, as defined by hospitalization, supplemental oxygen use, or death. Age 70 years and older was statistically significantly associated with a higher risk of developing severe COVID-19 disease.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5752-6212",
"affiliation": [
{
"name": "Department of Medicine, Memorial Sloan Kettering Cancer Center , New York, NY, USA"
},
{
"name": "Department of Medicine, Weill Cornell Medical Center , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Shah",
"given": "Neil J",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-2004-7298",
"affiliation": [
{
"name": "Department of Medicine, Icahn School of Medicine at Mount Sinai , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Patel",
"given": "Vaibhav G",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2796-3483",
"affiliation": [
{
"name": "Department of Medicine, Icahn School of Medicine at Mount Sinai , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Zhong",
"given": "Xiaobo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Columbia University Medical Center , New York, NY, USA"
}
],
"family": "Pina",
"given": "Luis",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1720-5654",
"affiliation": [
{
"name": "Department of Medicine, Columbia University Medical Center , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Hawley",
"given": "Jessica E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Montefiore Center for Cancer Care , Bronx, NY, USA"
}
],
"family": "Lin",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Montefiore Center for Cancer Care , Bronx, NY, USA"
}
],
"family": "Gartrell",
"given": "Benjamin A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4386-3490",
"affiliation": [
{
"name": "Department of Medicine, NYU Langone Medical Center , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Febles",
"given": "Victor Adorno",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3419-4723",
"affiliation": [
{
"name": "Department of Medicine, NYU Langone Medical Center , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Wise",
"given": "David R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Icahn School of Medicine at Mount Sinai , New York, NY, USA"
}
],
"family": "Qin",
"given": "Qian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8649-9855",
"affiliation": [
{
"name": "Department of Medicine, Icahn School of Medicine at Mount Sinai , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Mellgard",
"given": "George",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0272-0733",
"affiliation": [
{
"name": "Department of Medicine, Icahn School of Medicine at Mount Sinai , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Joshi",
"given": "Himanshu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2302-4171",
"affiliation": [
{
"name": "Department of Medicine, Weill Cornell Medical Center , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Nauseef",
"given": "Jones T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Weill Cornell Medical Center , New York, NY, USA"
}
],
"family": "Green",
"given": "David A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1704-1517",
"affiliation": [
{
"name": "Department of Medicine, Weill Cornell Medical Center , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Vlachostergios",
"given": "Panagiotis J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of California , San Francisco, CA, USA"
}
],
"family": "Kwon",
"given": "Daniel H",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of California , San Francisco, CA, USA"
}
],
"family": "Huang",
"given": "Franklin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Icahn School of Medicine at Mount Sinai , New York, NY, USA"
}
],
"family": "Liaw",
"given": "Bobby",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2777-8587",
"affiliation": [
{
"name": "Department of Medicine, Weill Cornell Medical Center , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Tagawa",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Memorial Sloan Kettering Cancer Center , New York, NY, USA"
},
{
"name": "Department of Medicine, Weill Cornell Medical Center , New York, NY, USA"
}
],
"family": "Kantoff",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Memorial Sloan Kettering Cancer Center , New York, NY, USA"
},
{
"name": "Department of Medicine, Weill Cornell Medical Center , New York, NY, USA"
}
],
"family": "Morris",
"given": "Michael J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5113-8147",
"affiliation": [
{
"name": "Department of Medicine, Icahn School of Medicine at Mount Sinai , New York, NY, USA"
}
],
"authenticated-orcid": false,
"family": "Oh",
"given": "William K",
"sequence": "additional"
}
],
"container-title": "JNCI Cancer Spectrum",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
6
]
],
"date-time": "2022-05-06T19:21:31Z",
"timestamp": 1651864891000
},
"deposited": {
"date-parts": [
[
2022,
6,
3
]
],
"date-time": "2022-06-03T13:25:25Z",
"timestamp": 1654262725000
},
"funder": [
{
"DOI": "10.13039/100000002",
"doi-asserted-by": "publisher",
"name": "NIH"
},
{
"DOI": "10.13039/100000054",
"doi-asserted-by": "publisher",
"name": "NCI"
}
],
"indexed": {
"date-parts": [
[
2022,
6,
3
]
],
"date-time": "2022-06-03T13:44:25Z",
"timestamp": 1654263865259
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
5,
2
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
5,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 10,
"start": {
"date-parts": [
[
2022,
5,
12
]
],
"date-time": "2022-05-12T00:00:00Z",
"timestamp": 1652313600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jncics/advance-article-pdf/doi/10.1093/jncics/pkac035/43691099/pkac035.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jncics/article-pdf/6/3/pkac035/43947547/pkac035.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jncics/article-pdf/6/3/pkac035/43947547/pkac035.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
5,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
12
]
]
},
"published-other": {
"date-parts": [
[
2022,
6,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
5,
2
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"key": "2022060313251268900_pkac035-B1",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "2022060313251268900_pkac035-B2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "2022060313251268900_pkac035-B3",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "2022060313251268900_pkac035-B4",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1128/JVI.02205-08",
"article-title": "Proteolytic activation of the 1918 influenza virus hemagglutinin",
"author": "Chaipan",
"doi-asserted-by": "crossref",
"first-page": "3200",
"issue": "7",
"journal-title": "J Virol",
"key": "2022060313251268900_pkac035-B5",
"volume": "83",
"year": "2009"
},
{
"DOI": "10.1128/JVI.01542-10",
"article-title": "Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "12658",
"issue": "24",
"journal-title": "J Virol",
"key": "2022060313251268900_pkac035-B6",
"volume": "84",
"year": "2010"
},
{
"DOI": "10.1128/JVI.01815-18",
"article-title": "TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection",
"author": "Iwata-Yoshikawa",
"doi-asserted-by": "crossref",
"first-page": "e01815",
"issue": "6",
"journal-title": "J Virol",
"key": "2022060313251268900_pkac035-B7",
"volume": "93",
"year": "2019"
},
{
"article-title": "Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2",
"author": "Lin",
"first-page": "4180",
"issue": "17",
"journal-title": "Cancer Res",
"key": "2022060313251268900_pkac035-B8",
"volume": "59",
"year": "1999"
},
{
"DOI": "10.1200/JCO.2012.48.6431",
"article-title": "Targeted androgen pathway suppression in localized prostate cancer: a pilot study",
"author": "Mostaghel",
"doi-asserted-by": "crossref",
"first-page": "229",
"issue": "3",
"journal-title": "J Clin Oncol",
"key": "2022060313251268900_pkac035-B9",
"volume": "32",
"year": "2014"
},
{
"DOI": "10.1016/j.mce.2009.12.022",
"article-title": "Androgen receptor and androgen-dependent gene expression in lung",
"author": "Mikkonen",
"doi-asserted-by": "crossref",
"first-page": "14",
"issue": "1–2",
"journal-title": "Mol Cell Endocrinol",
"key": "2022060313251268900_pkac035-B10",
"volume": "317",
"year": "2010"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n=4532)",
"author": "Montopoli",
"doi-asserted-by": "crossref",
"first-page": "1040",
"issue": "8",
"journal-title": "Ann. Oncol",
"key": "2022060313251268900_pkac035-B11",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.34330",
"article-title": "Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19",
"author": "Schmidt",
"doi-asserted-by": "crossref",
"first-page": "e2134330",
"issue": "11",
"journal-title": "JAMA Netw Open",
"key": "2022060313251268900_pkac035-B12",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.annonc.2020.06.005",
"article-title": "On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2",
"author": "Caffo",
"doi-asserted-by": "crossref",
"first-page": "1415",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "2022060313251268900_pkac035-B13",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.015",
"article-title": "Androgen deprivation and SARS-CoV-2 in men with prostate cancer",
"author": "Koskinen",
"doi-asserted-by": "crossref",
"first-page": "1417",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "2022060313251268900_pkac035-B14",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.023",
"article-title": "Does androgen deprivation therapy protect against severe complications from COVID-19?",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "1419",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "2022060313251268900_pkac035-B15",
"volume": "31",
"year": "2020"
},
{
"author": "World Health Organization",
"key": "2022060313251268900_pkac035-B16"
},
{
"DOI": "10.1097/JU.0000000000001338",
"article-title": "Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2",
"author": "Klein",
"doi-asserted-by": "crossref",
"first-page": "441",
"issue": "2",
"journal-title": "J Urol",
"key": "2022060313251268900_pkac035-B17",
"volume": "205",
"year": "2021"
},
{
"DOI": "10.1016/j.annonc.2021.01.067",
"article-title": "Androgen-deprivation therapy and SARS-CoV-2 in men with prostate cancer: findings from the University of California Health System registry",
"author": "Kwon",
"doi-asserted-by": "crossref",
"first-page": "678",
"issue": "5",
"journal-title": "Ann Oncol",
"key": "2022060313251268900_pkac035-B18",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-21171-x",
"article-title": "Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "866",
"issue": "1",
"journal-title": "Nat Commun",
"key": "2022060313251268900_pkac035-B19",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA",
"key": "2022060313251268900_pkac035-B20",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"article-title": "Factors associated with COVID-19-related death using OpenSAFELY",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "430",
"issue": "7821",
"journal-title": "Nature",
"key": "2022060313251268900_pkac035-B21",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "2022060313251268900_pkac035-B22",
"volume": "323",
"year": "2020"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jncics/article/doi/10.1093/jncics/pkac035/6584832"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cancer Research",
"Oncology"
],
"subtitle": [],
"title": "The Impact of Androgen Deprivation Therapy on COVID-19 Illness in Men With Prostate Cancer",
"type": "journal-article",
"volume": "6"
}
