Efficacy of a Persian herbal medicine compound on coronavirus disease 2019 (COVID-19): a randomized clinical trial
et al., Integrative Medicine Research, doi:10.1016/j.imr.2022.100869, IRCT20200330046899N1, Jun 2022
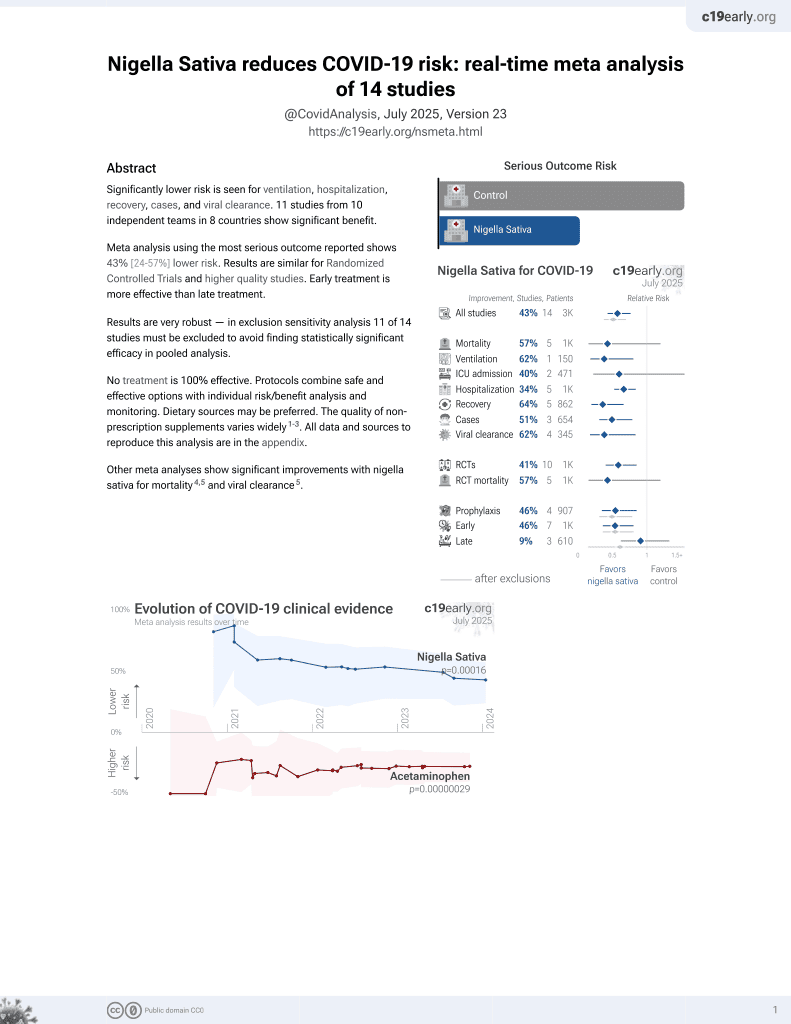
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
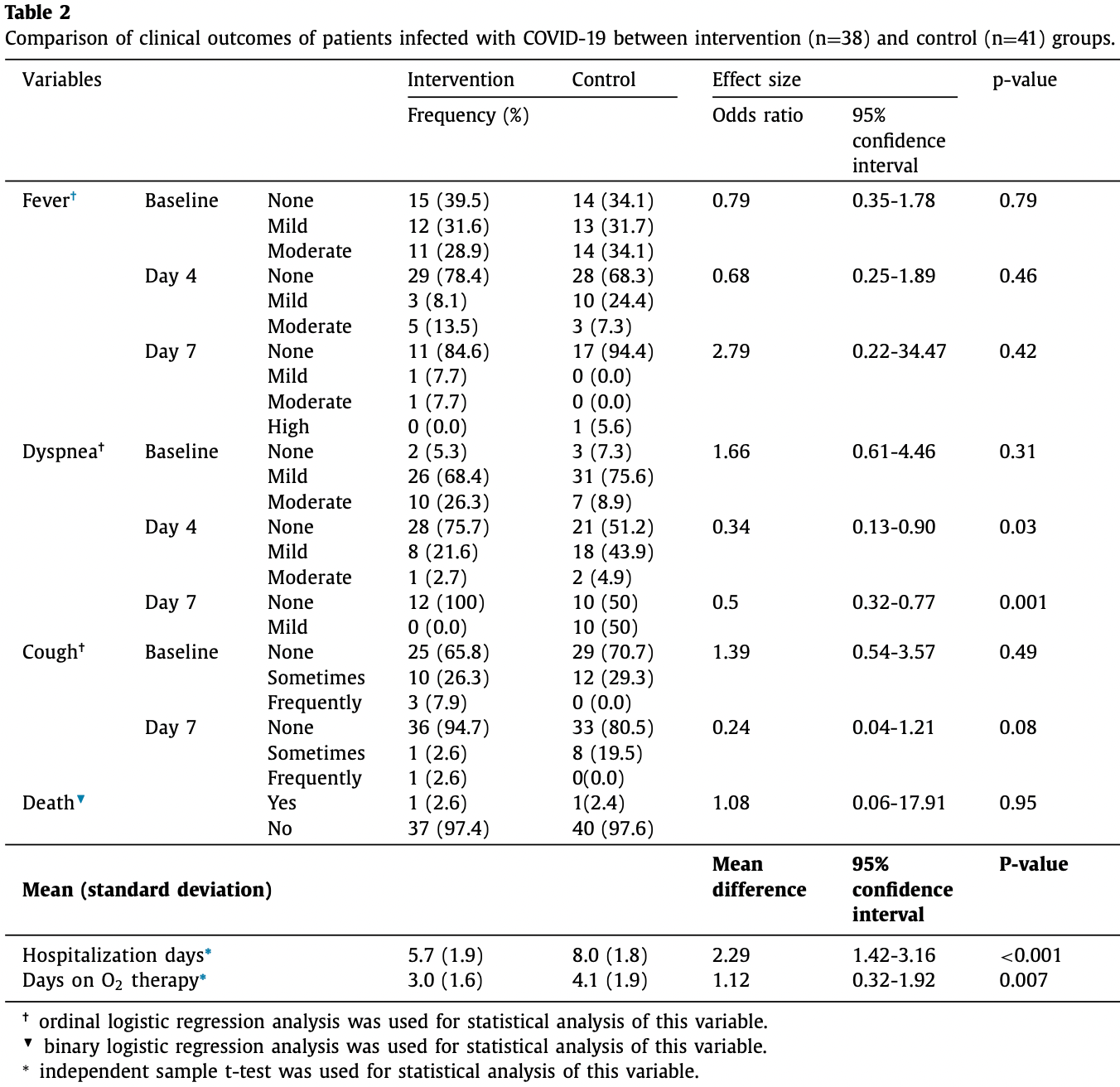
Small RCT 41 patients treated with nigella sativa, glycyrrhiza glabra, punica granatum, and rheum palmatum, and 41 control patients, showing shorter hospitalization with treatment.
|
risk of death, 7.9% higher, RR 1.08, p = 1.00, treatment 1 of 38 (2.6%), control 1 of 41 (2.4%).
|
|
oxygen time, 26.8% lower, relative time 0.73, p = 0.007, treatment mean 3.0 (±1.6) n=38, control mean 4.1 (±1.9) n=41.
|
|
hospitalization time, 28.7% lower, relative time 0.71, p < 0.001, treatment mean 5.7 (±1.9) n=38, control mean 8.0 (±1.8) n=41.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Setayesh et al., 3 Jun 2022, Randomized Controlled Trial, Iran, peer-reviewed, mean age 59.1, 7 authors, study period June 2020 - September 2020, this trial uses multiple treatments in the treatment arm (combined with glycyrrhiza glabra, punica granatum, and rheum palmatum) - results of individual treatments may vary, trial IRCT20200330046899N1.
Contact: hasheminasab@zaums.ac.ir, hashemifa67@gmail.com.
Efficacy of a Persian herbal medicine compound on coronavirus disease 2019 (COVID-19): A randomized controlled trial
Integrative Medicine Research, doi:10.1016/j.imr.2022.100869
Background: The global attention to the capacities of traditional medicine for alleviating the clinical manifestations of COVID-19 has been growing. The present trial aimed to evaluate the efficacy and safety of a Persian herbal medicine formula among patients with COVID-19. Methods: The present trial was conducted in Afzalipour hospital, Kerman, Iran, from June to September 2020. Hospitalized COVID-19 patients were randomly divided into intervention (Persian herbal medicine formula + routine treatment) or control (only routine treatment) groups. The intervention group received both capsule number 1 and 2 every 8 hours for 7 days. Capsule number 1 contained extract of the Glycyrrhiza glabra, Punica granatum , and Rheum palmatum , and the second capsule was filled by Nigella sativa powder. Participants were followed up to 7 days. The primary outcome was the number of hospitalization days, while cough, fever, and respiratory rate, days on oxygen (O 2 ) therapy, and mortality rate were considered as the secondary outcomes. Results: Eighty-two patients were enrolled to the study, while 79 cases completed the trial and their data were analyzed (mean age: 59.1 ± 17.1 years). Based on the results, the Persian medicine formula decreased the mean hospitalization days, so that the mean difference of length of hospitalization as primary outcome was 2.95 ± 0.43 days. A significant clinical improvement was observed regarding dyspnea, need for O 2 ) therapy, and respiratory rate in the intervention group. No adverse effects were reported.
Conclusion: The present study supported the use of the Persian medicine formula as an adjuvant therapy for hospitalized COVID-19 patients. Study registration: Iranian Registry of Clinical Trials (www.irct.ir): IRCT2020 0330 046899N1. Study registration: Iranian Registry of Clinical Trials (www.irct.ir): IRCT2020 0330 046899N1.
Conflict of interest There was no conflict of interest in this study.
CRediT authorship contribution statement
References
Azimi, Hashemi-Nasab, Mokaberinejad, Qaraaty, Mojahedi, The prevention and complementary therapy in Acute distress syndrome of COVID-19 in the viewpoint of Persian medicine: A narrative review, J Babol Univ Medical Sci, doi:10.22088/jbums.23.1.177
Azimi, Hasheminasab, Evaluating the efficacy and safety of the myrtle (Myrtus communis) in treatment and prognosis of patients suspected to novel coronavirus disease (COVID-19): study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-020-04915-w
Azimi, Mojahedi, Mokaberinejad, Hasheminasab, Ethnomedicine knowledge of Iranian traditional healers and the novel coronavirus disease 2019 (COVID-19), J Adv Med Med Res, doi:10.30699/jambs.29.135.238
Beketov, Pakhomov, Nesterova, Improved method of flavonoid extraction from bird cherry fruits, Pharm Chem J, doi:10.1007/s11094-005-0143-7
Bensaad, Kim, Quah, Kim, Shahimi, Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum, BMC Complement Altern Med, doi:10.1186/s12906-017-1555-0
Chandrasekaran, Deepak, Thiyagarajan, Kathiresan, Sangli et al., Dual inhibitory effect of Glycyrrhiza glabra (GutGardTM) on COX and LOX products, Phytomedicine, doi:10.1016/j.phymed.2010.08.001
Cinatl, Morgenstern, Bauer, Chandra, Rabenau et al., Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus, The Lancet, doi:10.1016/S0140-6736(03)13615-X
Cornélio Favarin, De Oliveira, Freire De Oliveira, De, Rogerio, Potential effects of medicinal plants and secondary metabolites on acute lung injury, Biomed Res Int, doi:10.1155/2013/576479
Forouzanfar, Bazzaz, Hosseinzadeh, Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects, Iran J Basic Med Sci, doi:10.22038/IJBMS.2015.3849
Franceschelli, Pesce, Vinciguerra, Ferrone, Riccioni et al., Licocalchone-C extracted from Glycyrrhiza glabra inhibits lipopolysaccharideinterferon-γ inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression, Molecules, doi:10.3390/molecules16075720
Frattaruolo, Carullo, Brindisi, Mazzotta, Bellissimo et al., Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L.(licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway, Antioxidants, doi:10.3390/antiox8060186
Ghannad, Mohammadi, Safiallahy, Faradmal, Azizi et al., The effect of aqueous extract of Glycyrrhiza glabra on herpes simplex virus 1, Jundishapur J Microbiol, doi:10.5812/jjm.11616
Guarner, Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19, Am J Clin Pathol, doi:10.1093/ajcp/aqaa029
Haidari, Ali, Casscells, Sw, Madjid, Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir, Phytomedicine, doi:10.1016/j.phymed.2009.06.002
Hashempur, Hashempour, Mosavat, Heydari, Rhazes-his life and contributions to the field of dermatology, JAMA Dermatol, doi:10.1001/jamadermatol.2016.0144
Ho, Wu, Chen, Li, Hsiang, Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction, Antivir Res, doi:10.1016/j.antiviral.2006.04.014
Hoever, Baltina, Michaelis, Kondratenko, Baltina et al., Antiviral activity of glycyrrhizic acid derivatives against SARS − coronavirus, J Med Chem, doi:10.1021/jm0493008
Hosseinzadeh, Bazzaz, Haghi, Antibacterial activity of total extracts and essential oil of Nigella sativa L. seeds in mice, Pharmacologyonline
Hosseinzadeh, Eskandari, Ziaee, Antitussive effect of thymoquinone, a constituent of Nigella sativa seeds, in guinea pigs, Pharmacologyonline
Iranzadasl, Karimi, Moadeli, Pasalar, Persian medicine recommendations for the prevention of pandemics related to the respiratory system: a narrative literature review, Integr Med Res, doi:10.1016/j.imr.2020.100483
Jin, Cai, Cheng, Cheng, Deng et al., A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version), Mil Med Res, doi:10.1186/s40779-020-0233-6
Johnson, Harris, Cain, Hummer, Goyal et al., Pulmonary and extra-pulmonary clinical manifestations of COVID-19, Front Med, doi:10.3389/fmed.2020.00526
Khan, Chemical composition of Nigella sativa Linn: part 2 recent advances, Inflammopharmacology, doi:10.1007/s10787-016-0262-7
Khorasani, Research Institute for Islamic and Complementary Medicine
Koshak, Koshak, Nigella sativa L. as a potential phytotherapy for covid-19: a mini-review of in-silico studies, Curr Ther Res Clin Exp, doi:10.1016/j.curtheres.2020.100602
Lin, Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro, Antivir Res, doi:10.1016/S0166-3542(03)00030-5
Liu, Gao, Yuan, Yang, Shi et al., Efficacy and safety of integrated traditional Chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis, Pharmacol Res, doi:10.1016/j.phrs.2020.104896
Luo, Su, Gong, Qin, Liu et al., Anti-SARS coronavirus 3C-like protease effects of Rheum palmatum L. extracts, Biosci Trends
Marinova, Ribarova, Atanassova, Total phenolics and total flavonoids in Bulgarian fruits and vegetables, J Chem Technol Metal
Marsik, Kokoska, Landa, Nepovim, Soudek et al., In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1-and-2-catalyzed prostaglandin E2 biosyntheses, Planta Med, doi:10.1055/s-2005-871288
Miyake, Tango, Ota, Mitamura, Yoshiba et al., Efficacy of Stronger Neo-Minophagen C compared between two doses administered three times a week on patients with chronic viral hepatitis, J Gastroenterol Hepatol, doi:10.1046/j.1440-1746.2002.02876.x
Mohit, Farrokhzad, Faraji, Heidarzadeh-Esfahani, Kafeshani, Effect of Nigella sativa L. supplementation on inflammatory and oxidative stress indicators: a systematic review and meta-analysis of controlled clinical trials, Complement Ther Med, doi:10.1016/j.ctim.2020.102535
Pal, Berhanu, Desalegn, Kandi, Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): An update, Cureus, doi:10.7759/cureus.7423
Parvaiz, Hussain, Khalid, Hussnain, Iram et al., A review: Medicinal importance of Glycyrrhiza glabra L.(fabaceae family), Global J Pharmacol, doi:10.5829/idosi.gjp.2014.8.1.81179
Qiu, Wei, Zhao, Zhong, Zhao et al., Outcome reporting from protocols of clinical trials of coronavirus disease 2019 (COVID-19): a review, medRxiv, doi:10.1101/2020.03.04.20031401
Rahman, Jahan, Defining a 'Risk Group'and Ageism in the Era of COVID-19, J Loss Trauma, doi:10.1080/15325024.2020.1757993
Salem, Hossain, Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection, Int J Immunopharmacol, doi:10.1016/S0192-0561(00)00036-9
Schubert, Lansky, Neeman, Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids, J Ethnopharmacol, doi:10.1016/s0378-8741(98)00222-0
Sekhavati, Jafari, Seyedalinaghi, Jamalimoghadamsiahkali, Sadr et al., Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106143
Shakeri, Hashempur, Beigomi, Khiveh, Nejatbakhsh et al., Strategies in traditional Persian medicine to maintain a healthy life in the elderly, J Complement Integr Med, doi:10.1515/jcim-2019-0273
Shakeri, Hashempur, Mojibian, Aliasl, Bioos et al., A comparative study of ranitidine and quince (Cydonia oblonga mill) sauce on gastroesophageal reflux disease (GERD) in pregnancy: a randomised, open-label, active-controlled clinical trial, J Obstet Gynaecol, doi:10.1080/01443615.2018.1431210
Shaygannia, Bahmani, Zamanzad, Rafieian-Kopaei, A review study on Punica granatum L, J Evid Based Complementary Altern Med, doi:10.1177/2156587215598039
Siahpoosh, How can Persian medicine (traditional Iranian medicine) be effective to control COVID-19?, Trad Integr Med, doi:10.18502/tim.v5i2.3624
Soleymani, Zargaran, A historical report on preparing sustained release dosage forms for addicts in medieval Persia, 16th Century AD. Subst Use Misuse, doi:10.1080/10826084.2018.1432648
Song, Li, Wu, -X, Shen, Liu et al., Emodin alleviates alternatively activated macrophage and asthmatic airway inflammation in a murine asthma model, Acta Pharmacol Sin, doi:10.1038/aps.2017.147
Song, Wang, Zhang, Investigation of urinary interleukin-6 level in chronic renal failure patients and the influence of Rheum palmatum in treating it, Zhongguo Zhong Xi Yi Jie He Za Zhi
Sultan, Buttxs, Qayyum, Suleria, Immunity: plants as effective mediators, Crit Rev Food Sci Nutr, doi:10.1080/10408398.2011.633249
Tajmiri, Farhangi, Dehghan, Nigella Sativa treatment and serum concentrations of thyroid hormones, transforming growth factor β (TGF-β) and interleukin 23 (IL-23) in patients with Hashimoto's thyroiditis, Eur J Integr Med, doi:10.1016/j.eujim.2016.03.003
Thiyagarajan, Chandrasekaran, Deepak, Agarwal, Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents, Inflammopharmacology, doi:10.1007/s10787-011-0080-x
Vardanjani, Heydari, Dowran, Pasalar, A cross-sectional study of Persian medicine and the COVID-19 pandemic in Iran: Rumors and recommendations, Integr Med Res, doi:10.1016/j.imr.2020.100482
Walsh, Effectiveness of Chinese herbal medicine and Persian medicine against viral infections: A systematic review, Syst Rev Pharm, doi:10.31838/srp.2021.2.6
Zhang, Qiu, Wang, Long, Mao, Effect of Rheum palmatum L. on NF-κB signaling pathway of mice with acute liver failure, Asian Pac J Trop Med, doi:10.1016/j.apjtm.2015.09.011
Zhang, Zhan, Yao, Gao, Shong, Antiviral activity of tannin from the pericarp of Punica granatum L. against genital Herpes virus in vitro, China J Chin Mater Med
DOI record:
{
"DOI": "10.1016/j.imr.2022.100869",
"ISSN": [
"2213-4220"
],
"URL": "http://dx.doi.org/10.1016/j.imr.2022.100869",
"alternative-id": [
"S2213422022000373"
],
"article-number": "100869",
"author": [
{
"affiliation": [],
"family": "Setayesh",
"given": "Mohammad",
"sequence": "first"
},
{
"affiliation": [],
"family": "Karimi",
"given": "Mehrdad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zargaran",
"given": "Arman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abousaidi",
"given": "Hamid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shahesmaeili",
"given": "Armita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amiri",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hasheminasab",
"given": "Fatemeh Sadat",
"sequence": "additional"
}
],
"container-title": "Integrative Medicine Research",
"container-title-short": "Integrative Medicine Research",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
4
]
],
"date-time": "2022-06-04T01:02:52Z",
"timestamp": 1654304572000
},
"deposited": {
"date-parts": [
[
2022,
6,
4
]
],
"date-time": "2022-06-04T01:03:33Z",
"timestamp": 1654304613000
},
"indexed": {
"date-parts": [
[
2022,
6,
4
]
],
"date-time": "2022-06-04T01:41:29Z",
"timestamp": 1654306889954
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
1
]
],
"date-time": "2022-06-01T00:00:00Z",
"timestamp": 1654041600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 2,
"start": {
"date-parts": [
[
2022,
6,
3
]
],
"date-time": "2022-06-03T00:00:00Z",
"timestamp": 1654214400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213422022000373?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213422022000373?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100869",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
6
]
]
},
"published-print": {
"date-parts": [
[
2022,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Effectiveness of Chinese Herbal Medicine and Persian Medicine against Viral Infections: A systematic Review",
"author": "Walsh",
"first-page": "65",
"issue": "2",
"journal-title": "Systematic Reviews in Pharmacy",
"key": "10.1016/j.imr.2022.100869_bib0001",
"volume": "12",
"year": "2020"
},
{
"author": "Guarner",
"key": "10.1016/j.imr.2022.100869_bib0002",
"series-title": "Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19",
"year": "2020"
},
{
"article-title": "Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): An update",
"author": "Pal",
"issue": "3",
"journal-title": "Cureus",
"key": "10.1016/j.imr.2022.100869_bib0003",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1186/s40779-020-0233-6",
"article-title": "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "4",
"issue": "1",
"journal-title": "Military Medical Research",
"key": "10.1016/j.imr.2022.100869_bib0004",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2020.00526",
"article-title": "Pulmonary and extra-pulmonary clinical manifestations of COVID-19",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "526",
"journal-title": "Frontiers in Medicine",
"key": "10.1016/j.imr.2022.100869_bib0005",
"volume": "7",
"year": "2020"
},
{
"article-title": "Defining a ‘Risk Group'and Ageism in the Era of COVID-19",
"author": "Rahman",
"first-page": "1",
"journal-title": "Journal of Loss and Trauma",
"key": "10.1016/j.imr.2022.100869_bib0006",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04915-w",
"article-title": "Evaluating the efficacy and safety of the myrtle (Myrtus communis) in treatment and prognosis of patients suspected to novel coronavirus disease (COVID-19): study protocol for a randomized controlled trial",
"author": "Azimi",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Trials",
"key": "10.1016/j.imr.2022.100869_bib0007",
"volume": "21",
"year": "2020"
},
{
"article-title": "Outcome reporting from protocols of clinical trials of Coronavirus Disease 2019 (COVID-19): a review",
"author": "Qiu",
"journal-title": "medRxiv",
"key": "10.1016/j.imr.2022.100869_bib0008",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106143",
"article-title": "Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial",
"author": "Sekhavati",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "International journal of antimicrobial agents",
"key": "10.1016/j.imr.2022.100869_bib0009",
"volume": "56",
"year": "2020"
},
{
"article-title": "The Prevention and Complementary Therapy in Acute Distress Syndrome of COVID-19 in the Viewpoint of Persian Medicine: A Narrative Review",
"author": "Azimi",
"journal-title": "Journal of Babol University of Medical Sciences",
"key": "10.1016/j.imr.2022.100869_bib0010",
"year": "2022"
},
{
"DOI": "10.30699/jambs.29.135.238",
"article-title": "Ethnomedicine Knowledge of Iranian Traditional Healers and the Novel Coronavirus Disease 2019 (COVID-19)",
"author": "Azimi",
"doi-asserted-by": "crossref",
"first-page": "238",
"issue": "135",
"journal-title": "Journal of Advances in Medical and Biomedical Research",
"key": "10.1016/j.imr.2022.100869_bib0011",
"volume": "29",
"year": "2021"
},
{
"article-title": "Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a systematic review and meta-analysis",
"author": "Liu",
"journal-title": "Pharmacological Research",
"key": "10.1016/j.imr.2022.100869_bib0012",
"year": "2020"
},
{
"DOI": "10.1080/10826084.2018.1432648",
"article-title": "A Historical Report on Preparing Sustained Release Dosage Forms for Addicts in Medieval Persia, 16th Century AD",
"author": "Soleymani",
"doi-asserted-by": "crossref",
"first-page": "1726",
"issue": "10",
"journal-title": "Substance Use & Misuse",
"key": "10.1016/j.imr.2022.100869_bib0013",
"volume": "53",
"year": "2018"
},
{
"article-title": "Strategies in traditional Persian medicine to maintain a healthy life in the elderly",
"author": "Shakeri",
"issue": "ahead-of-print",
"journal-title": "Journal of Complementary and Integrative Medicine",
"key": "10.1016/j.imr.2022.100869_bib0014",
"volume": "1",
"year": "2020"
},
{
"article-title": "Persian medicine recommendations for the prevention of pandemics related to the respiratory system: a narrative literature review",
"author": "Iranzadasl",
"journal-title": "Integrative Medicine Research",
"key": "10.1016/j.imr.2022.100869_bib0015",
"year": "2020"
},
{
"key": "10.1016/j.imr.2022.100869_bib0016",
"unstructured": "Siahpoosh MB. How Can Persian Medicine (Traditional Iranian Medicine) Be Effective to Control COVID-19? Traditional and Integrative Medicine 2020."
},
{
"DOI": "10.1016/j.imr.2020.100482",
"article-title": "A cross-sectional study of Persian medicine and the COVID-19 pandemic in Iran: Rumors and recommendations",
"author": "Vardanjani",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "Integrative medicine research",
"key": "10.1016/j.imr.2022.100869_bib0017",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1001/jamadermatol.2016.0144",
"article-title": "Rhazes—his life and contributions to the field of dermatology",
"author": "Hashempur",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "JAMA dermatology",
"key": "10.1016/j.imr.2022.100869_bib0018",
"volume": "153",
"year": "2017"
},
{
"DOI": "10.1080/01443615.2018.1431210",
"article-title": "A comparative study of ranitidine and quince (Cydonia oblonga mill) sauce on gastroesophageal reflux disease (GERD) in pregnancy: a randomised, open-label, active-controlled clinical trial",
"author": "Shakeri",
"doi-asserted-by": "crossref",
"first-page": "899",
"issue": "7",
"journal-title": "Journal of Obstetrics and Gynaecology",
"key": "10.1016/j.imr.2022.100869_bib0019",
"volume": "38",
"year": "2018"
},
{
"DOI": "10.1007/s10787-016-0262-7",
"article-title": "Chemical composition of Nigella sativa Linn: part 2 recent advances",
"author": "Khan",
"doi-asserted-by": "crossref",
"first-page": "67",
"issue": "2-3",
"journal-title": "Inflammopharmacology",
"key": "10.1016/j.imr.2022.100869_bib0020",
"volume": "24",
"year": "2016"
},
{
"article-title": "Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects",
"author": "Forouzanfar",
"first-page": "929",
"issue": "12",
"journal-title": "Iranian journal of basic medical sciences",
"key": "10.1016/j.imr.2022.100869_bib0021",
"volume": "17",
"year": "2014"
},
{
"article-title": "Antitussive effect of thymoquinone, a constituent of Nigella sativa seeds, in guinea pigs",
"author": "Hosseinzadeh",
"first-page": "480",
"journal-title": "Pharmacologyonline",
"key": "10.1016/j.imr.2022.100869_bib0022",
"volume": "2",
"year": "2008"
},
{
"article-title": "Antibacterial activity of total extracts and essential oil of Nigella sativa L. seeds in mice",
"author": "Hosseinzadeh",
"first-page": "429",
"journal-title": "Pharmacologyonline",
"key": "10.1016/j.imr.2022.100869_bib0023",
"volume": "2",
"year": "2007"
},
{
"DOI": "10.1016/S0192-0561(00)00036-9",
"article-title": "Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection",
"author": "Salem",
"doi-asserted-by": "crossref",
"first-page": "729",
"issue": "9",
"journal-title": "International journal of immunopharmacology",
"key": "10.1016/j.imr.2022.100869_bib0024",
"volume": "22",
"year": "2000"
},
{
"article-title": "A review: Medicinal importance of Glycyrrhiza glabra L.(Fabaceae family)",
"author": "Parvaiz",
"first-page": "8",
"issue": "1",
"journal-title": "Global J Pharmacol",
"key": "10.1016/j.imr.2022.100869_bib0025",
"volume": "8",
"year": "2014"
},
{
"DOI": "10.1016/S0140-6736(03)13615-X",
"article-title": "Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus",
"author": "Cinatl",
"doi-asserted-by": "crossref",
"first-page": "2045",
"issue": "9374",
"journal-title": "The Lancet",
"key": "10.1016/j.imr.2022.100869_bib0026",
"volume": "361",
"year": "2003"
},
{
"DOI": "10.1021/jm0493008",
"article-title": "Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS− Coronavirus",
"author": "Hoever",
"doi-asserted-by": "crossref",
"first-page": "1256",
"issue": "4",
"journal-title": "Journal of medicinal chemistry",
"key": "10.1016/j.imr.2022.100869_bib0027",
"volume": "48",
"year": "2005"
},
{
"DOI": "10.1016/S0166-3542(03)00030-5",
"article-title": "Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "1",
"journal-title": "Antiviral research",
"key": "10.1016/j.imr.2022.100869_bib0028",
"volume": "59",
"year": "2003"
},
{
"DOI": "10.1046/j.1440-1746.2002.02876.x",
"article-title": "Efficacy of Stronger Neo-Minophagen C compared between two doses administered three times a week on patients with chronic viral hepatitis",
"author": "Miyake",
"doi-asserted-by": "crossref",
"first-page": "1198",
"issue": "11",
"journal-title": "Journal of gastroenterology and hepatology",
"key": "10.1016/j.imr.2022.100869_bib0029",
"volume": "17",
"year": "2002"
},
{
"article-title": "The effect of aqueous extract of Glycyrrhiza glabra on herpes simplex virus 1",
"author": "Ghannad",
"issue": "7",
"journal-title": "Jundishapur journal of microbiology",
"key": "10.1016/j.imr.2022.100869_bib0030",
"volume": "7",
"year": "2014"
},
{
"DOI": "10.1080/10408398.2011.633249",
"article-title": "Immunity: plants as effective mediators",
"author": "Sultan",
"doi-asserted-by": "crossref",
"first-page": "1298",
"issue": "10",
"journal-title": "Critical reviews in food science and nutrition",
"key": "10.1016/j.imr.2022.100869_bib0031",
"volume": "54",
"year": "2014"
},
{
"DOI": "10.1177/2156587215598039",
"article-title": "A review study on Punica granatum L",
"author": "Shaygannia",
"doi-asserted-by": "crossref",
"first-page": "221",
"issue": "3",
"journal-title": "Journal of evidence-based complementary & alternative medicine",
"key": "10.1016/j.imr.2022.100869_bib0032",
"volume": "21",
"year": "2016"
},
{
"DOI": "10.1155/2013/576479",
"article-title": "Potential effects of medicinal plants and secondary metabolites on acute lung injury",
"author": "Cornélio Favarin",
"doi-asserted-by": "crossref",
"journal-title": "BioMed research international 2013",
"key": "10.1016/j.imr.2022.100869_bib0033",
"year": "2013"
},
{
"DOI": "10.1016/S0378-8741(98)00222-0",
"article-title": "Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids",
"author": "Schubert",
"doi-asserted-by": "crossref",
"first-page": "11",
"issue": "1",
"journal-title": "Journal of ethnopharmacology",
"key": "10.1016/j.imr.2022.100869_bib0034",
"volume": "66",
"year": "1999"
},
{
"DOI": "10.1016/j.phymed.2009.06.002",
"article-title": "Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir",
"author": "Haidari",
"doi-asserted-by": "crossref",
"first-page": "1127",
"issue": "12",
"journal-title": "Phytomedicine",
"key": "10.1016/j.imr.2022.100869_bib0035",
"volume": "16",
"year": "2009"
},
{
"article-title": "Antiviral activity of tannin from the pericarp of Punica granatum L. against genital Herpes virus in vitro. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi=",
"author": "Zhang",
"first-page": "556",
"issue": "9",
"journal-title": "China journal of Chinese materia medica",
"key": "10.1016/j.imr.2022.100869_bib0036",
"volume": "20",
"year": "1995"
},
{
"article-title": "Anti-SARS coronavirus 3C-like protease effects of Rheum palmatum L. extracts",
"author": "Luo",
"issue": "4",
"journal-title": "Bioscience trends",
"key": "10.1016/j.imr.2022.100869_bib0037",
"volume": "3",
"year": "2009"
},
{
"DOI": "10.1016/j.antiviral.2006.04.014",
"article-title": "Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction",
"author": "Ho",
"doi-asserted-by": "crossref",
"first-page": "92",
"issue": "2",
"journal-title": "Antiviral research",
"key": "10.1016/j.imr.2022.100869_bib0038",
"volume": "74",
"year": "2007"
},
{
"DOI": "10.1038/aps.2017.147",
"article-title": "Emodin alleviates alternatively activated macrophage and asthmatic airway inflammation in a murine asthma model",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "1317",
"issue": "8",
"journal-title": "Acta Pharmacologica Sinica",
"key": "10.1016/j.imr.2022.100869_bib0039",
"volume": "39",
"year": "2018"
},
{
"article-title": "Total phenolics and total flavonoids in Bulgarian fruits and vegetables",
"author": "Marinova",
"first-page": "255",
"issue": "3",
"journal-title": "Journal of the university of chemical technology and metallurgy",
"key": "10.1016/j.imr.2022.100869_bib0040",
"volume": "40",
"year": "2005"
},
{
"DOI": "10.1007/s11094-005-0143-7",
"article-title": "Improved method of flavonoid extraction from bird cherry fruits",
"author": "Beketov",
"doi-asserted-by": "crossref",
"first-page": "316",
"issue": "6",
"journal-title": "Pharmaceutical Chemistry Journal",
"key": "10.1016/j.imr.2022.100869_bib0041",
"volume": "39",
"year": "2005"
},
{
"DOI": "10.1016/j.phymed.2010.08.001",
"article-title": "Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products",
"author": "Chandrasekaran",
"doi-asserted-by": "crossref",
"first-page": "278",
"issue": "4",
"journal-title": "Phytomedicine",
"key": "10.1016/j.imr.2022.100869_bib0042",
"volume": "18",
"year": "2011"
},
{
"DOI": "10.3390/antiox8060186",
"article-title": "Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L.(licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway",
"author": "Frattaruolo",
"doi-asserted-by": "crossref",
"first-page": "186",
"issue": "6",
"journal-title": "Antioxidants",
"key": "10.1016/j.imr.2022.100869_bib0043",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.3390/molecules16075720",
"article-title": "Licocalchone-C extracted from Glycyrrhiza glabra inhibits lipopolysaccharide-interferon-γ inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression",
"author": "Franceschelli",
"doi-asserted-by": "crossref",
"first-page": "5720",
"issue": "7",
"journal-title": "Molecules",
"key": "10.1016/j.imr.2022.100869_bib0044",
"volume": "16",
"year": "2011"
},
{
"DOI": "10.1007/s10787-011-0080-x",
"article-title": "Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents",
"author": "Thiyagarajan",
"doi-asserted-by": "crossref",
"first-page": "235",
"issue": "4",
"journal-title": "Inflammopharmacology",
"key": "10.1016/j.imr.2022.100869_bib0045",
"volume": "19",
"year": "2011"
},
{
"DOI": "10.1016/j.apjtm.2015.09.011",
"article-title": "Effect of Rheum palmatum L. on NF-κB signaling pathway of mice with acute liver failure",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "841",
"issue": "10",
"journal-title": "Asian Pacific journal of tropical medicine",
"key": "10.1016/j.imr.2022.100869_bib0046",
"volume": "8",
"year": "2015"
},
{
"article-title": "Investigation of urinary interleukin-6 level in chronic renal failure patients and the influence of Rheum palmatum in treating it",
"author": "Song",
"first-page": "107",
"issue": "2",
"journal-title": "Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi Jiehe Zazhi= Chinese Journal of Integrated Traditional and Western Medicine",
"key": "10.1016/j.imr.2022.100869_bib0047",
"volume": "20",
"year": "2000"
},
{
"DOI": "10.1186/s12906-017-1555-0",
"article-title": "Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum",
"author": "BenSaad",
"doi-asserted-by": "crossref",
"first-page": "47",
"issue": "1",
"journal-title": "BMC complementary and alternative medicine",
"key": "10.1016/j.imr.2022.100869_bib0048",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1016/j.ctim.2020.102535",
"article-title": "Effect of Nigella sativa L. supplementation on inflammatory and oxidative stress indicators: A systematic review and meta-analysis of controlled clinical trials",
"author": "Mohit",
"doi-asserted-by": "crossref",
"journal-title": "Complementary Therapies in Medicine",
"key": "10.1016/j.imr.2022.100869_bib0049",
"year": "2020"
},
{
"DOI": "10.1016/j.eujim.2016.03.003",
"article-title": "Nigella Sativa treatment and serum concentrations of thyroid hormones, transforming growth factor β (TGF-β) and interleukin 23 (IL-23) in patients with Hashimoto's Thyroiditis",
"author": "Tajmiri",
"doi-asserted-by": "crossref",
"first-page": "576",
"issue": "4",
"journal-title": "European Journal of Integrative Medicine",
"key": "10.1016/j.imr.2022.100869_bib0050",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1055/s-2005-871288",
"article-title": "In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1-and-2-catalyzed prostaglandin E2 biosyntheses",
"author": "Marsik",
"doi-asserted-by": "crossref",
"first-page": "739",
"issue": "08",
"journal-title": "Planta medica",
"key": "10.1016/j.imr.2022.100869_bib0051",
"volume": "71",
"year": "2005"
},
{
"article-title": "Nigella sativa l. as a potential phytotherapy for covid-19: A mini-review of in-silico studies",
"author": "Koshak",
"journal-title": "Current Therapeutic Research",
"key": "10.1016/j.imr.2022.100869_bib0052",
"year": "2020"
},
{
"author": "Aghili Khorasani",
"key": "10.1016/j.imr.2022.100869_bib0053",
"series-title": "Makhzan ul-Advia",
"year": "2001"
}
],
"reference-count": 53,
"references-count": 53,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213422022000373"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Complementary and alternative medicine"
],
"subtitle": [],
"title": "Efficacy of a Persian herbal medicine compound on coronavirus disease 2019 (COVID-19): a randomized clinical trial",
"type": "journal-article"
}