Antiviral efficacy of oral ensitrelvir versus oral ritonavir-boosted nirmatrelvir in COVID-19
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(25)00482-7, PLATCOV, NCT05041907, May 2025 (preprint)
RCT 604 low-risk adults with early COVID-19 symptoms showing significantly improved SARS-CoV-2 viral clearance with both ensitrelvir and paxlovid.
Inclusion criteria selected for low-risk patients with high viral loads which may not generalize to high-risk patients or patients treated prior to having high viral load.
Authors indicate that "between March 2023 and April 2024 the three study arms randomised 604 patients", however figure S7 shows paxlovid patients starting before July 2022.
120 day long COVID results in the protocol are not reported.
The protocol was modified mid-trial in October 2024: the number of patients was increased, the swabbing schedule was changed, and patient self-swabbing was added. Results appear to have improved in the post-change period.
The swabbing schedule in the paper conflicts with the October 2024 change reported in the protocol ("now swabbing day 0 to day 5 once or twice per day to characterise viral clearance").
Study covers ensitrelvir and paxlovid.
|
risk of hospitalization, 5.0% lower, RR 0.95, p = 1.00, treatment 1 of 201 (0.5%), control 1 of 191 (0.5%), NNT 3839, COVID-19 related.
|
|
risk of hospitalization, 42.5% higher, RR 1.43, p = 1.00, treatment 3 of 201 (1.5%), control 2 of 191 (1.0%), all cause.
|
|
recovery time, 8.5% lower, relative time 0.92, p = 0.006, treatment mean 5.4 (±1.81) n=201, control mean 5.9 (±1.76) n=191, all.
|
|
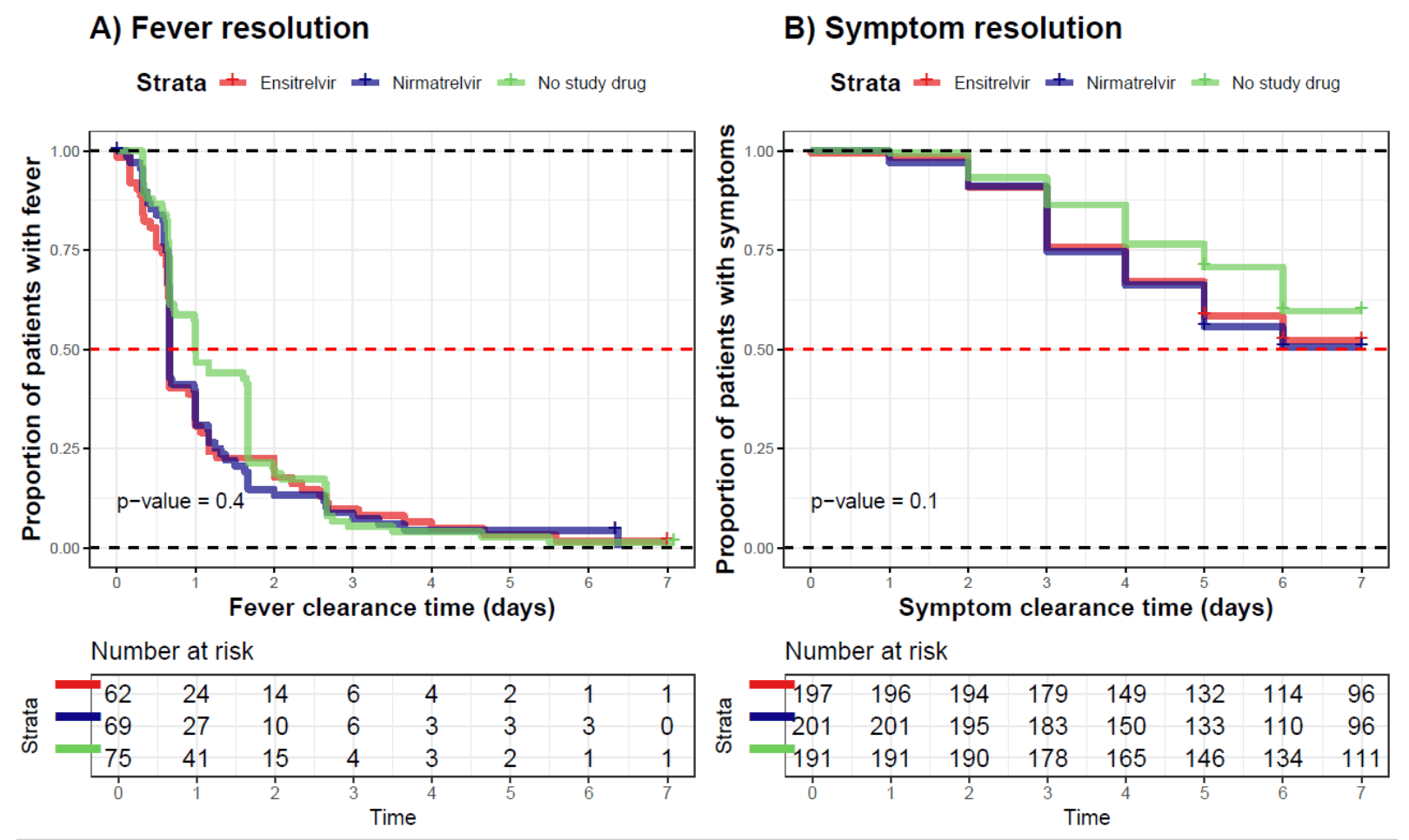
recovery time, 14.3% lower, relative time 0.86, p = 0.37, treatment mean 1.2 (±2.53) n=201, control mean 1.4 (±1.76) n=191, fever.
|
|
relative clearance half-life, 55.2% better, RR 0.45, p < 0.001, treatment median 5.2 IQR 2.8 n=201, control median 11.6 IQR 6.4 n=191, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Schilling et al., 21 May 2025, Randomized Controlled Trial, multiple countries, peer-reviewed, median age 31.0, 37 authors, study period 17 March, 2023 - 21 April, 2024, average treatment delay 1.8 days, trial NCT05041907 (history) (PLATCOV).
Contact: william@tropmedres.ac.
Antiviral efficacy of oral ensitrelvir versus oral ritonavir-boosted nirmatrelvir in COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(25)00482-7
Background Ensitrelvir is an oral antiviral treatment for COVID-19 with the same molecular target (the main protease) as ritonavir-boosted nirmatrelvir-the current oral first-line treatment. We aimed to compare the clinical antiviral effects of the two drugs.
Methods In an open-label, phase 2, randomised, controlled, adaptive pharmacometric platform trial, low-risk adult outpatients aged 18-60 years with early symptomatic COVID-19 (<4 days of symptoms) were recruited from hospital acute respiratory infection clinics in Thailand and Laos. Patients were randomly assigned in blocks (block sizes depended on the number of interventions available) to one of eight treatment groups, including oral ensitrelvir and oral ritonavirboosted nirmatrelvir at standard doses, both given for 5 days, and no study drug. The primary endpoint was the oropharyngeal SARS-CoV-2 viral clearance rate assessed between day 0 and day 5 in the modified intention-to-treat population (defined as patients with at least 2 days of follow-up). Patients had four oropharyngeal swabs taken on day 0 and two swabs taken daily from days 1 to 7, then on days 10 and 14. Viral clearance rates were derived under a Bayesian hierarchical linear model fitted to log 10 viral densities in standardised paired oropharyngeal swab eluates taken daily over the 5 days (14 samples). An individual patient data meta-analysis of all small molecule drugs evaluated in this platform trial using published results was also performed, adjusting for temporal trends in viral clearance. This trial is registered at ClinicalTrials.gov, NCT05041907. Findings Between March 17, 2023, and April 21, 2024, 604 of 903 patients enrolled were concurrently assigned to the three treatment groups (ensitrelvir n=202; ritonavir-boosted nirmatrelvir n=207; no study drug n=195). Median estimated SARS-CoV-2 clearance half-lives were 5•9 h (IQR 4•0-8•6) with ensitrelvir, 5•2 h (3•8-6•6) with nirmatrelvir, and 11•6 h (8•1-14•5) with no study drug. Viral clearance following ensitrelvir was 82% faster (95% credible interval 61-104) than no study drug and 16% slower (5-25) than ritonavir-boosted nirmatrelvir. In the metaanalysis of all unblinded small molecule drugs evaluated in the platform trial, nirmatrelvir and ensitrelvir had the largest antiviral effects (1157 patients). Viral rebound occurred in 15 (7%) of 207 patients in the nirmatrelvir group and 10 (5%) of 202 in the ensitrelvir group (p=0•45). Interpretation Both ensitrelvir and nirmatrelvir accelerate oropharyngeal SARS-CoV-2 viral clearance. Ensitrelvir is an effective alternative to currently available antivirals in treating COVID-19. Although COVID-19 is now generally a mild disease, it still causes substantial morbidity, particularly in vulnerable groups, and new variants or other coronaviruses could still emerge with pandemic potential. Safe effective and affordable antivirals are needed, and these are best assessed initially in pharmacometric platform trials assessing..
References
Boyd, Singh, Schilling, White, Evidence that remdesivir treatment reduces viral titers in patients with COVID-19, Antimicrob Agents Chemother
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
De Grooth, Geenen, Girbes, Vincent, Parienti et al., SOFA and mortality endpoints in randomized controlled trials: a systematic review and metaregression analysis, Crit Care
Elias, Khan, Stadler, Viral clearance as a surrogate of clinical efficacy for COVID-19 therapies in outpatients: a systematic review and meta-analysis, Lancet Microbe
Hammond, Fountaine, Yunis, Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Jittamala, Boyd, Schilling, Antiviral efficacy of fluoxetine in early symptomatic COVID-19: an open-label, randomised, controlled, adaptive platform trial (PLATCOV), eClinicalMedicine
Jittamala, Schilling, Watson, Clinical antiviral efficacy of remdesivir and casirivimab/imdevimab against the SARS-CoV-2 Delta and Omicron variants, medRxiv, doi:10.1101/2022.10.17.22281161(preprint
Jittamala, Schilling, Watson, Clinical antiviral efficacy of remdesivir in coronavirus disease 2019: an open-label, randomized controlled adaptive platform trial (PLATCOV), J Infect Dis
Luetkemeyer, Chew, Lacey, Ensitrelvir for the treatment of nonhospitalized adults with COVID-19: results from the SCORPIO-HR, phase 3, randomized, double-blind, placebocontrolled trial, Clin Infect Dis
Luvira, Schilling, Jittamala, Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial, BMC Infect Dis
Mateja, Chu, Murray, The choice of viral load end point in early phase trials of COVID-19 treatments aiming to reduce 28-day hospitalization and/or death, J Infect Dis
Sanderson, Hisner, Donovan-Banfield, A molnupiravirassociated mutational signature in global SARS-CoV-2 genomes, Nature
Schilling, Jittamala, Watson, Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial, Lancet Infect Dis
Schilling, Jittamala, Watson, Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, controlled adaptive platform trial (PLATCOV), eLife
Singh, Boyd, Schilling, Watson, Mukaka et al., The relationship between viral clearance rates and disease progression in early symptomatic COVID-19: a systematic review and meta-regression analysis, J Antimicrob Chemother
Unoh, Uehara, Nakahara, Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19, J Med Chem
Usher, The global COVID-19 treatment divide, Lancet
Watson, Kissler, Day, Grad, White, Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies, Antimicrob Agents Chemother
Wongnak, Schilling, Jittamala, Temporal changes in SARS-CoV-2 clearance kinetics and the optimal design of antiviral pharmacodynamic studies: an individual patient data meta-analysis of a randomised, controlled, adaptive platform study (PLATCOV), Lancet Infect Dis
Yotsuyanagi, Ohmagari, Doi, Efficacy and safety of 5-day oral ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial, JAMA Netw Open
DOI record:
{
"DOI": "10.1016/s1473-3099(25)00482-7",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(25)00482-7",
"alternative-id": [
"S1473309925004827"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Antiviral efficacy of oral ensitrelvir versus oral ritonavir-boosted nirmatrelvir in COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(25)00482-7"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(25)00549-3"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Schilling",
"given": "William H K",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jittamala",
"given": "Podjanee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wongnak",
"given": "Phrutsamon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watson",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyd",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luvira",
"given": "Viravarn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siripoon",
"given": "Tanaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngamprasertchai",
"given": "Thundon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Batty",
"given": "Elizabeth M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beer",
"given": "Ellen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Shivani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asawasriworanan",
"given": "Tanatchakorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seers",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Phommasone",
"given": "Koukeo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Terry John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kruabkontho",
"given": "Varaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngernseng",
"given": "Thatsanun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tubprasert",
"given": "Jaruwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdad",
"given": "Mohammad Yazid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madmanee",
"given": "Wanassanan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kouhathong",
"given": "Jindarat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suwannasin",
"given": "Kanokon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pagornrat",
"given": "Watcharee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piteekan",
"given": "Tianrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanboonkunupakarn",
"given": "Borimas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poovorawan",
"given": "Kittiyod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Potaporn",
"given": "Manus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Srisubat",
"given": "Attasit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loharjun",
"given": "Bootsakorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chotivanich",
"given": "Kesinee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imwong",
"given": "Mallika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pukrittayakamee",
"given": "Sasithon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dondorp",
"given": "Arjen M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Day",
"given": "Nicholas P J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piyaphanee",
"given": "Watcharapong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Phumratanaprapin",
"given": "Weerapong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "White",
"given": "Nicholas J",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.com",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.jp",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
11
]
],
"date-time": "2025-10-11T22:29:45Z",
"timestamp": 1760221785000
},
"deposited": {
"date-parts": [
[
2025,
10,
11
]
],
"date-time": "2025-10-11T22:29:48Z",
"timestamp": 1760221788000
},
"funder": [
{
"DOI": "10.13039/100010269",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100010269",
"id-type": "DOI"
}
],
"name": "Wellcome Trust"
}
],
"indexed": {
"date-parts": [
[
2025,
10,
11
]
],
"date-time": "2025-10-11T23:11:39Z",
"timestamp": 1760224299058,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
1
]
],
"date-time": "2025-10-01T00:00:00Z",
"timestamp": 1759276800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
1
]
],
"date-time": "2025-10-01T00:00:00Z",
"timestamp": 1759276800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
8
]
],
"date-time": "2025-09-08T00:00:00Z",
"timestamp": 1757289600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309925004827?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309925004827?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
10
]
]
},
"published-print": {
"date-parts": [
[
2025,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(22)00372-5",
"article-title": "The global COVID-19 treatment divide",
"author": "Usher",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(25)00482-7_bib1",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00493-0",
"article-title": "Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial",
"author": "Schilling",
"doi-asserted-by": "crossref",
"first-page": "36",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(25)00482-7_bib2",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1038/s41586-023-06649-6",
"article-title": "A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes",
"author": "Sanderson",
"doi-asserted-by": "crossref",
"first-page": "594",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(25)00482-7_bib3",
"volume": "623",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(25)00482-7_bib4",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.2c00117",
"article-title": "Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19",
"author": "Unoh",
"doi-asserted-by": "crossref",
"first-page": "6499",
"journal-title": "J Med Chem",
"key": "10.1016/S1473-3099(25)00482-7_bib5",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1186/s13054-017-1609-1",
"article-title": "SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis",
"author": "de Grooth",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "Crit Care",
"key": "10.1016/S1473-3099(25)00482-7_bib6",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.1093/jac/dkae045",
"article-title": "The relationship between viral clearance rates and disease progression in early symptomatic COVID-19: a systematic review and meta-regression analysis",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "935",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/S1473-3099(25)00482-7_bib7",
"volume": "79",
"year": "2024"
},
{
"DOI": "10.1016/S2666-5247(23)00398-1",
"article-title": "Viral clearance as a surrogate of clinical efficacy for COVID-19 therapies in outpatients: a systematic review and meta-analysis",
"author": "Elias",
"doi-asserted-by": "crossref",
"first-page": "e459",
"journal-title": "Lancet Microbe",
"key": "10.1016/S1473-3099(25)00482-7_bib8",
"volume": "5",
"year": "2024"
},
{
"DOI": "10.7554/eLife.83201",
"article-title": "Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, controlled adaptive platform trial (PLATCOV)",
"author": "Schilling",
"doi-asserted-by": "crossref",
"journal-title": "eLife",
"key": "10.1016/S1473-3099(25)00482-7_bib9",
"volume": "12",
"year": "2023"
},
{
"article-title": "Clinical antiviral efficacy of remdesivir and casirivimab/imdevimab against the SARS-CoV-2 Delta and Omicron variants",
"author": "Jittamala",
"journal-title": "medRxiv",
"key": "10.1016/S1473-3099(25)00482-7_bib10",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiad275",
"article-title": "Clinical antiviral efficacy of remdesivir in coronavirus disease 2019: an open-label, randomized controlled adaptive platform trial (PLATCOV)",
"author": "Jittamala",
"doi-asserted-by": "crossref",
"first-page": "1318",
"journal-title": "J Infect Dis",
"key": "10.1016/S1473-3099(25)00482-7_bib11",
"volume": "228",
"year": "2023"
},
{
"DOI": "10.1186/s12879-023-08835-3",
"article-title": "Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial",
"author": "Luvira",
"doi-asserted-by": "crossref",
"first-page": "89",
"journal-title": "BMC Infect Dis",
"key": "10.1016/S1473-3099(25)00482-7_bib12",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1128/aac.01266-24",
"article-title": "Evidence that remdesivir treatment reduces viral titers in patients with COVID-19",
"author": "Boyd",
"doi-asserted-by": "crossref",
"first-page": "e01266",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/S1473-3099(25)00482-7_bib13",
"volume": "68",
"year": "2024"
},
{
"DOI": "10.1016/S1473-3099(24)00183-X",
"article-title": "Temporal changes in SARS-CoV-2 clearance kinetics and the optimal design of antiviral pharmacodynamic studies: an individual patient data meta-analysis of a randomised, controlled, adaptive platform study (PLATCOV)",
"author": "Wongnak",
"doi-asserted-by": "crossref",
"first-page": "953",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(25)00482-7_bib14",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1128/aac.00192-22",
"article-title": "Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies",
"author": "Watson",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/S1473-3099(25)00482-7_bib15",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2024.103036",
"article-title": "Antiviral efficacy of fluoxetine in early symptomatic COVID-19: an open-label, randomised, controlled, adaptive platform trial (PLATCOV)",
"author": "Jittamala",
"doi-asserted-by": "crossref",
"journal-title": "eClinicalMedicine",
"key": "10.1016/S1473-3099(25)00482-7_bib16",
"volume": "80",
"year": "2025"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(25)00482-7_bib17",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2309003",
"article-title": "Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1186",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(25)00482-7_bib18",
"volume": "390",
"year": "2024"
},
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"article-title": "Efficacy and safety of 5-day oral ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/S1473-3099(25)00482-7_bib19",
"volume": "7",
"year": "2024"
},
{
"DOI": "10.1093/infdis/jiaf282",
"article-title": "The choice of viral load end point in early phase trials of COVID-19 treatments aiming to reduce 28-day hospitalization and/or death",
"author": "Mateja",
"doi-asserted-by": "crossref",
"first-page": "60",
"journal-title": "J Infect Dis",
"key": "10.1016/S1473-3099(25)00482-7_bib20",
"volume": "232",
"year": "2025"
},
{
"DOI": "10.1093/cid/ciaf029",
"article-title": "Ensitrelvir for the treatment of nonhospitalized adults with COVID-19: results from the SCORPIO-HR, phase 3, randomized, double-blind, placebo-controlled trial",
"author": "Luetkemeyer",
"doi-asserted-by": "crossref",
"first-page": "1235",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S1473-3099(25)00482-7_bib21",
"volume": "80",
"year": "2025"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309925004827"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Antiviral efficacy of oral ensitrelvir versus oral ritonavir-boosted nirmatrelvir in COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}
schilling4