Efficacy of oral indomethacin in the treatment of COVID-19 infection; a randomized clinical trial
et al., Immunopathologia Persa, doi:10.34172/ipp.2022.xx, IRCT20200427047215N1, Jan 2022
Very small RCT with 22 indomethacin and 23 control patients, showing no significant difference in outcomes. All patients were treated with HCQ.
|
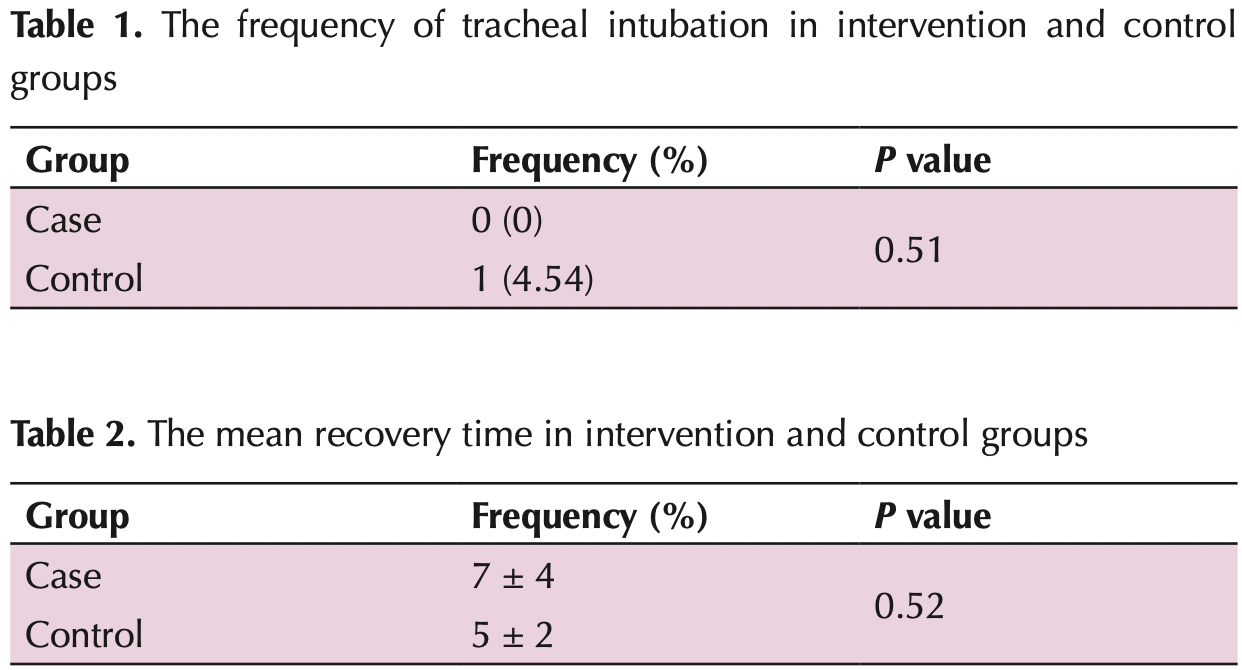
risk of mechanical ventilation, 66.2% lower, RR 0.34, p = 1.00, treatment 0 of 22 (0.0%), control 1 of 23 (4.3%), NNT 23, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
recovery time, 40.0% higher, relative time 1.40, p = 0.52, treatment 22, control 23.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Salmasi et al., 13 Jan 2022, Randomized Controlled Trial, Iran, peer-reviewed, 8 authors, trial IRCT20200427047215N1.
Efficacy of oral indomethacin in the treatment of COVID-19 infection; a randomized clinical trial
doi:10.34172/ipp.2022.xx.
Introduction: Given the anti-viral activity of indomethacin and that it may be as a potent inhibitor of coronavirus replication, the goal of this study was to assess the efficacy of oral indomethacin in the treatment of COVID-19 infection. Objectives: We evaluated the efficacy of oral Indomethacin on pneumonia of COVID 19 infection. Patients and Methods: This randomized clinical trial was conducted on 45 patients with moderate symptoms of coronavirus-induced pneumonia admitted to Amin and Noor hospitals. All patients were randomly divided into two groups. In the intervention and control groups, 200 mg of hydroxychloroquine tablet was administered twice daily for 5 days. Acetaminophen was also prescribed if needed. Moreover, 75 mg indomethacin in slow-release formulation was administered for 5 days in the intervention group. Then patients were assessed regarding clinical parameters.
Results: The mean age of patients in the case and control groups was 51.59 ± 15.74 and 56.65 ± 12.90 years old, respectively (P = 0.41). Among 45 patients, 22 (48.9%) and 23 patients (51.1%) were male and female, respectively. The frequency of tracheal intubation in intervention and control groups was 0 (0%) and 1(4.54%), respectively (P = 0.51). The mean recovery time in the intervention and control groups was 7 ± 4 and 5 ± 2, respectively (P = 0.52). Furthermore, no patients in the two groups were re-hospitalized up to 28 days (P > 0.05). Moreover, there was no significant difference between the two groups after intervention in terms of SpO2% (P = 02). Conclusion: According to these findings, oral indomethacin did not affect tracheal intubation in patients with COVID-19. Moreover, mean recovery time, re-hospitalization and SpO2 value were not influenced by indomethacin. Therefore, the use of oral indomethacin is not suggested in these patients. Trial Registration: The trial protocol was approved by the Iranian Registry of Clinical Trials website(identifier: IRCT20200427047215N1; https://www.irct.ir/trial/47520, ethical code; IR.MUI.MED.REC.1399.045).
Authors' contribution MS, SP and AD were the principal investigators of the study. MS, AD, FS, and BA were included in preparing the concept and design. AD and ZA revisited the manuscript and critically evaluated the intellectual contents. All authors participated in preparing the final draft of the manuscript, revised the manuscript and critically evaluated the intellectual contents. All authors have read and approved the content of the manuscript and confirmed the accuracy or integrity of any part of the work.
Conflicts of interest The authors declare that they have no competing interests.
Ethical issues The research was conducted in accordance with the tents of the Declaration of Helsinki. The Ethics Committee of Isfahan University of Medical Sciences approved this study (IR.MUI.MED.REC.1399.045). Accordingly, written informed consent was taken from all participants before any intervention. The trial protocol was approved by the Iranian Registry of Clinical Trials (identifier: IRCT20200427047215N1; https://www.irct.ir/trial/47520). Moreover, Ethical issues (including plagiarism, data fabrication, double publication) have been completely observed by the authors. This study was a part of internal medicine residency thesis of Zeinab Ahmadikia at this university.
Funding/Support None.
References
Alkotaji, Al-Zidan, Indomethacin: can it counteract bradykinin effects in COVID-19 patients?, Curr Pharmacol Rep, doi:10.1007/s40495-021-00257-6
Amici, Caro, Ciucci, Chiappa, Castilletti et al., Indomethacin has a potent antiviral activity against SARS coronavirus, Antivir Ther
Amici, Frazia, Brunelli, Balsamo, Angelini et al., Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR, Cell Microbiol
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Kanakaraj, Ravichandran, Low Dose Indomethacin in the Outpatient Treatment of COVID-19 in Kidney Transplant Recipients-A Case Series, Open Access Libr J, doi:10.4236/oalib.1106860
Li, Guan, Wu, Wang, Zhou et al., Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia, N Engl J Med
Lund, Reilev, Hallas, Kristensen, Thomsen et al., Association of Nonsteroidal Antiinflammatory Drug Use and Adverse Outcomes Among Patients Hospitalized With Influenza, JAMA Netw Open
Marinella, Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19, Int J Clin Pract
Raaben, Einerhand, Taminiau, Van Houdt, Bouma et al., Cyclooxygenase activity is important for efficient replication of mouse hepatitis virus at an early stage of infection, Virol J
Ravichandran, Purnab, Vijayaraghavaluc, Kalavakollub, Gaidhaned et al., Efficacy and safety of Indomethacin in Covid-19 patients, doi:medRxiv.2020.10.1101/2020.12.14.20245266
Reynolds, Enquist, Biological interactions between herpesviruses and cyclooxygenase enzymes, Rev Med Virol
Shimizu, Mochizuki, Shigeta, Morikawa, Effect of inhaled indomethacin on exercise-induced bronchoconstriction in children with asthma, Am J Respir Crit Care Med
Tu, Tu, Gao, Shao, Sheng, Current epidemiological and clinical features of COVID-19; a global perspective from China, J Infect, doi:10.1016/j.jinf.2020.04.011