The Impact of Colchicine on COVID-19 patients: A Clinical Trial Study
et al., Mediterranean Journal of Rheumatology, doi:10.31138/mjr.33.2.232, IRCT20200418047126N1, Sep 2020 (preprint)
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
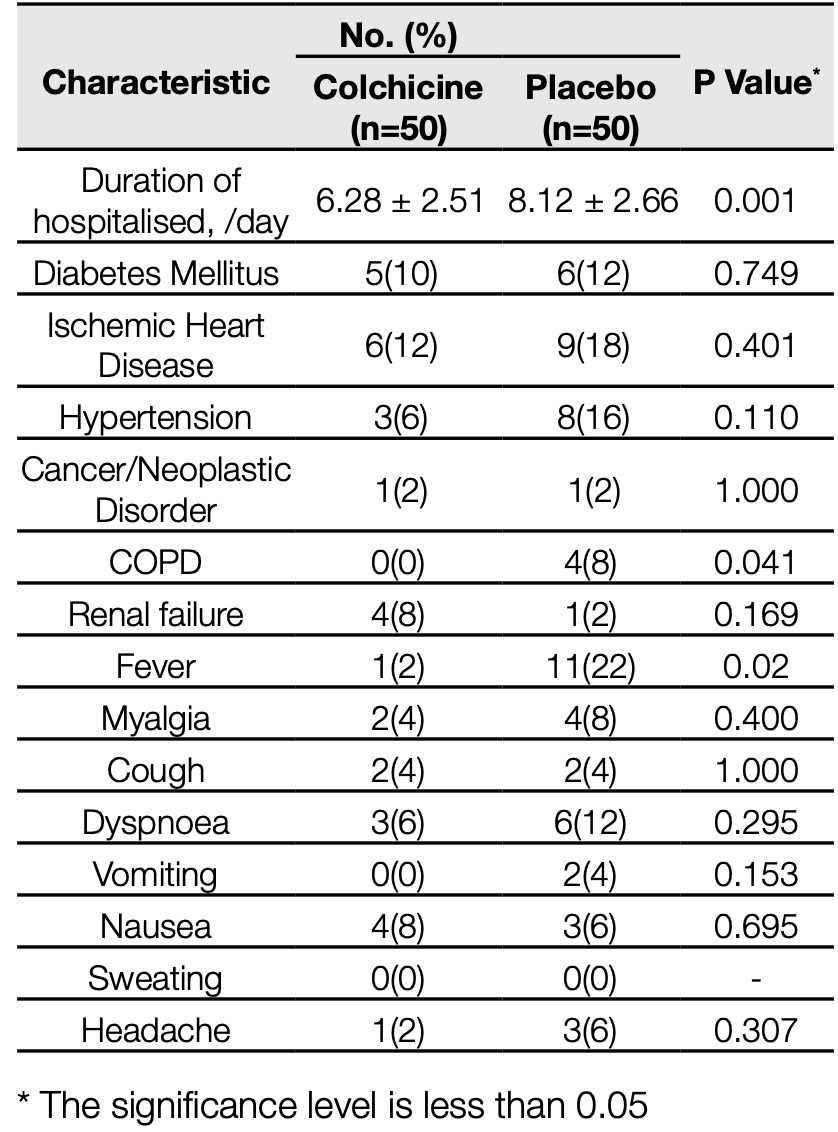
Open label RCT with 100 hospitalized patients in Iran, 50 treated with colchicine, showing shorter hospitalization time with treatment. There were no deaths.
|
hospitalization time, 22.7% lower, relative time 0.77, p = 0.001, treatment 50, control 50.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Salehzadeh et al., 21 Sep 2020, Randomized Controlled Trial, Iran, peer-reviewed, median age 56.0, 3 authors, study period 21 May, 2020 - 20 June, 2020, average treatment delay 6.28 (treatment) 8.12 (control) days, trial IRCT20200418047126N1.
Contact: sobhan13295@gmail.com.
The Impact of Colchicine on COVID-19 patients: A Clinical Trial Study
Mediterranean Journal of Rheumatology, doi:10.31138/mjr.33.2.232
Acute respiratory distress syndrome COVID-19: Coronavirus Disease 2019 ICU: Intensive care unit IL: Interleukin BACKGROUND In late 2019, several cases of an acute respiratory illness (now known as the new coronavirus or COVID-19) were reported in Wuhan, China. [1] [2] [3] The coronavirus has spread rapidly to all over the world. As of Dec. 1, 2020, a total of over 61.8 million people infected by the virus and caused 1.4 million deaths all over the world. 4 There are various reports on the pathophysiology of the disease. Many studies have suggested that an overreaction of the immune system by virus ©Salehzadeh F, Pourfarzi F, Ataei S.
Custom Trial registration ID is: 47707 (irct.ir). The written confirmed consent obtained from all participants. Current Controlled prospective Trials registration ID that has been approved by ICMJE and WHO ICTRP registry is IRCT20200418047126N1, and the date of registration is 2020-05-14.
CONSENT FOR PUBLICATION Authors have taken written informed consent for this work.
CONFLICT OF INTEREST The authors declare no conflict of interest.
FUNDING Authors declare no private funding in this study.
AUTHOR CONTRIBUTIONS All authors have read and approved the manuscript and they contribute as: FP, Worked on epidemiologic aspects of the study; FS designed the study and wrote draft copy, SA collected all data and wrote the final copy.
References
Buonaguro, Puzanov, Ascierto, Anti-IL6R role in treatment of COVID-19-related ARDS, J Transl Med
Cascella, Rajnik, Cuomo, Dulebohn, Napoli, Features, Evaluation and Treatment Coronavirus (COVID-19)
Chen, Xiong, Bao, Shi, Convalescent plasma as a potential therapy for COVID-19, Lancet Infect Dis
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki et al., Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Netw Open
Gautret, Lagier, Parola, Hoang Thuan, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial
Hui, Azhar, Madani, Ntoumi, Kock et al., The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health: the latest 2019 novel corona cytokine virus outbreak in Wuhan
Lu, Stratton, Tang, Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle
Mansouri, Marjani, Tabarsi, Garnier, Mansouri, Successful Treatment of Covid-19 Associated Cytokine Release Syndrome with Colchicine. A Case Report and Review of Literature, Immunological Investigations, Immunol Invest
Martínez, Robertson, Barraclough, Xia, Mallat et al., Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome, J Am Heart Assoc
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Robertson, Martínez, Payet, Barraclough, Celermajer et al., Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation, Clin Sci (Lond)
Slobodnick, Shah, Krasnokutsky, Pillinger, Update on colchicine, Rheumatology
Tanaka, Narazaki, Kishimoto, Immunotherapeutic implications of IL-6 blockade for cytokine storm, Immunotherapy
Tisoncik, Korth, Simmons, Farrar, Martin et al., None, Microbiol Mol Biol Rev Mar
Vardhana, Wolchok, The many faces of the anti-COVID immune response, J Exp Med
Vitiello, Ferrara, Pelliccia, Granata, Porta, Cytokine storm and colchicine potential role fighting SARS-CoV-2 pneumonia, Ital J Med
Zhang, Wu, Li, Zhao, Wang, The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality, Int J Antimicrob Agents
DOI record:
{
"DOI": "10.31138/mjr.33.2.232",
"ISSN": [
"2529-198X"
],
"URL": "http://dx.doi.org/10.31138/mjr.33.2.232",
"alternative-id": [
"file382_1566"
],
"author": [
{
"affiliation": [
{
"name": "Pediatric Department, Bouali Children`s Hospital, Ardabil University of Medical Sciences (ARUMS), Ardabil, Iran,"
}
],
"family": "Salehzadeh",
"given": "Farhad",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Community Medicine, Ardabil University of Medical Sciences (ARUMS), Ardabil, Iran,"
}
],
"family": "Pourfarzi",
"given": "Farhad",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Pediatric Department, Bouali Children’s Hospital, Ardabil University of Medical Sciences (ARUMS), Ardabil, Iran"
}
],
"family": "Ataei",
"given": "Sobhan",
"sequence": "first"
}
],
"container-title": "Mediterranean Journal of Rheumatology",
"container-title-short": "MJR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T15:36:34Z",
"timestamp": 1662478594000
},
"deposited": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T15:59:00Z",
"timestamp": 1662479940000
},
"indexed": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T16:42:58Z",
"timestamp": 1662482578742
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
6
]
]
}
},
"language": "en",
"member": "16240",
"original-title": [],
"page": "232",
"prefix": "10.31138",
"published": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Convin SA",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.mjrheum.org/assets/files/792/file382_1566.pdf"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Rheumatology"
],
"subtitle": [],
"title": "The Impact of Colchicine on COVID-19 patients: A Clinical Trial Study",
"type": "journal-article",
"volume": "33"
}
