Ropeginterferon Alfa-2b is a long-acting, mono-pegylated recombinant Type I interferon biologic administered via subcutaneous injection.
May 21 2024 |
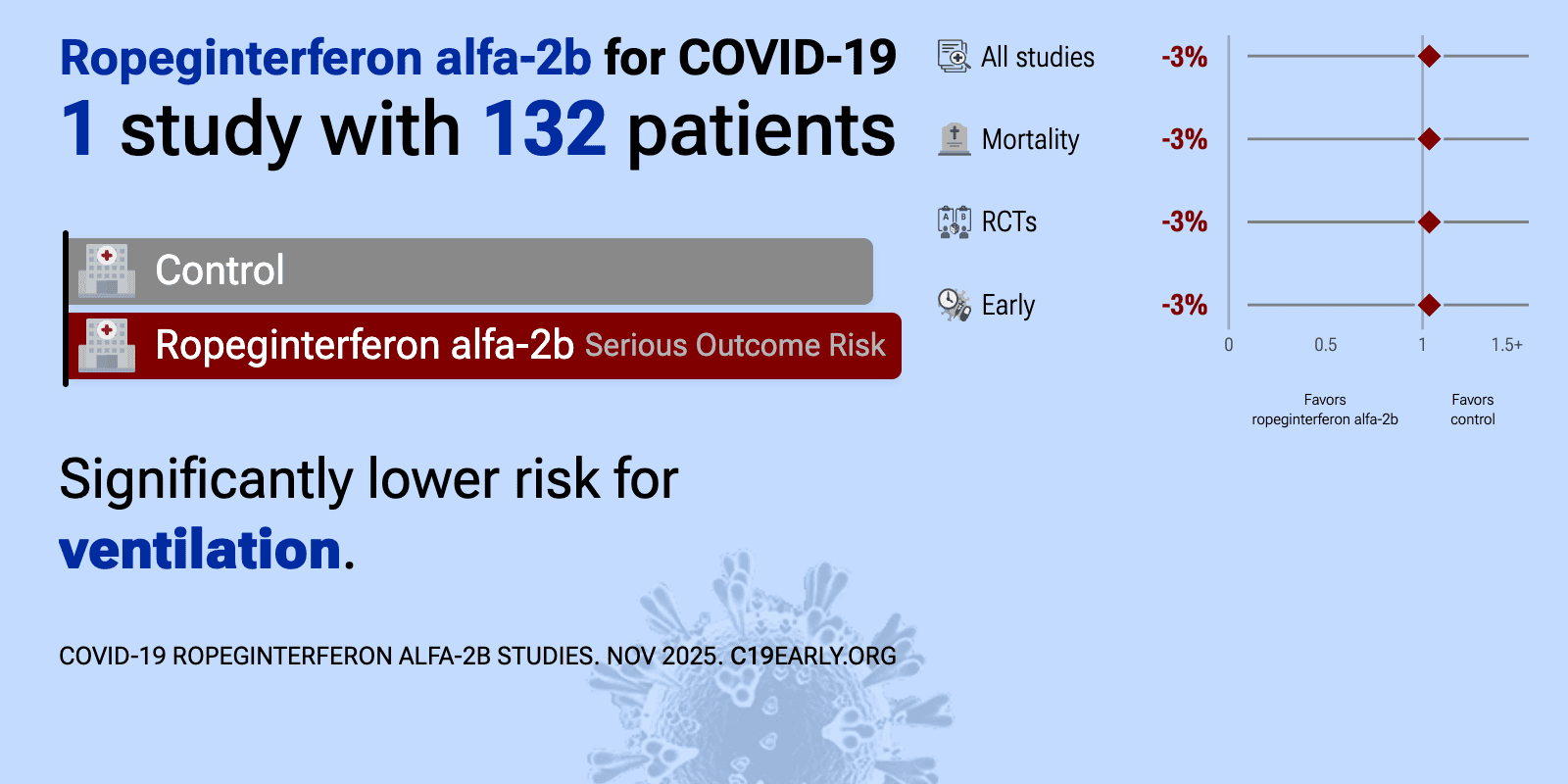
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-024-00992-5 | A Phase 3, Randomized, Controlled Trial Evaluating the Efficacy and Safety of Ropeginterferon Alfa-2b in Patients with Moderate COVID-19 |
| 90% lower ventilation (p=0.04), 3% higher need for oxygen therapy (p=1), and 4% improved recovery (p=1). RCT 132 hospitalized moderate COVID-19 patients in Taiwan showing higher rates of viral clearance or discharge by day 11 with ropeginterferon alfa-2b. The ropeginterferon alfa-2b group also showed higher rates of improvement in lung infil.. | ||