Mast cells and histamine receptor-targeted adjunctive treatments for COVID-19: A literature review
, D., Innovative Medicines & Omics, doi:10.36922/IMO025440058, Feb 2026
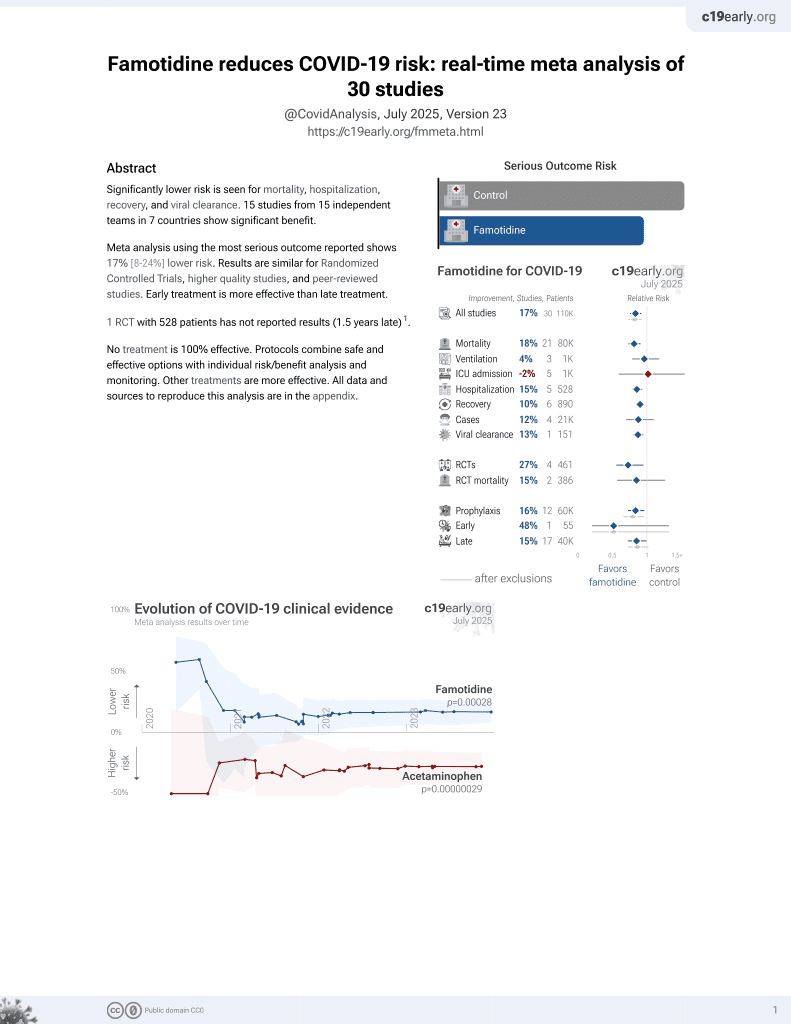
Famotidine for COVID-19
28th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
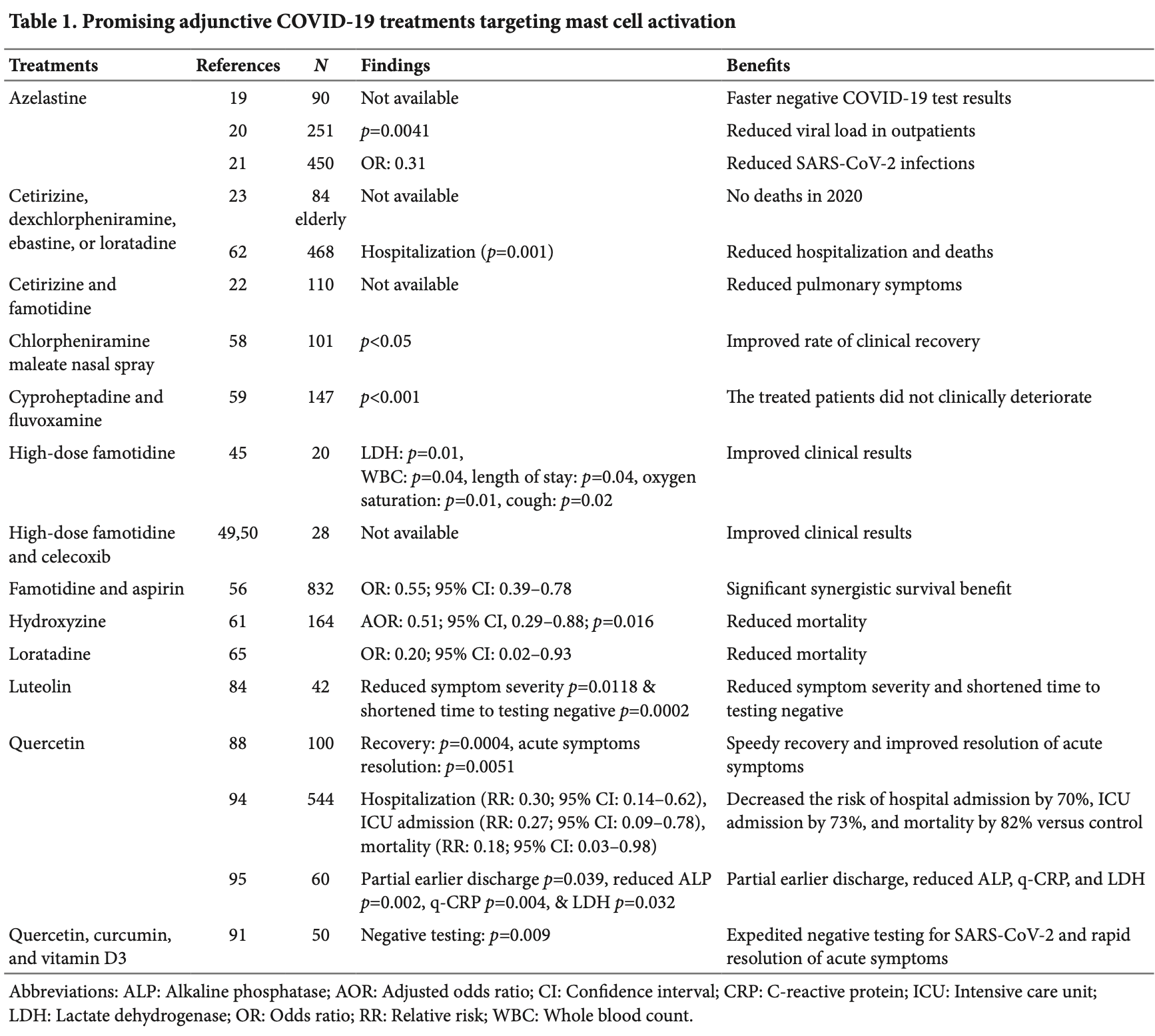
Review of clinical studies on antihistamines, mast cell stabilizers, and leukotriene receptor antagonists for COVID-19 treatment. Author finds that several mast cell-targeting agents show clinical benefits, with quercetin emerging as one of the most consistently supported treatment. Author reports that high-dose famotidine benefited COVID-19 patients, while standard heartburn doses showed mixed results, and that high-dose famotidine combined with celecoxib was associated with significant clinical, biomarker, and renal function improvements. Author notes that azelastine was associated with reduced viral load and lower risk of SARS-CoV-2 infection, and that hydroxyzine use within 48 hours of hospitalization was associated with reduced mortality. Author also highlights very good results with antihistamines (dexchlorpheniramine, cetirizine, loratadine, or ebastine) combined with azithromycin.
1.
Ricke, D., Mast cells and histamine receptor-targeted adjunctive treatments for COVID-19: A literature review, Innovative Medicines & Omics, doi:10.36922/IMO025440058.
Ricke et al., 4 Feb 2026, peer-reviewed, 1 author.
Abstract: Innovative Medicines & Omics
REVIEW ARTICLE
Mast cells and histamine receptor-targeted
adjunctive treatments for COVID-19: A literature
review
Darrell O. Ricke*
Department of Research, Molecular BioInsights, Winchester, Massachusetts, United States of
America
Abstract
*Corresponding author:
Darrell Ricke
(doricke@molecularbioinsights.
com)
Citation: Ricke DO. Mast cells
and histamine receptor-targeted
adjunctive treatments for
COVID-19: A literature review.
Innov Med Omics.
doi: 10.36922/IMO025440058
Received: October 27, 2025
Revised: November 20, 2025
Accepted: December 8, 2025
Published online: February 4,
2026
Copyright: © 2026 Author(s).
This is an Open-Access article
distributed under the terms of the
Creative Commons Attribution
License, permitting distribution,
and reproduction in any medium,
provided the original work is
properly cited.
Publisher’s Note: AccScience
Publishing remains neutral with
regard to jurisdictional claims in
published maps and institutional
affiliations.
Volume X Issue X (2026)
With the rollout of multiple COVID-19 vaccines, adjunctive treatments for COVID-19
have received less attention. Breakthrough infections post-vaccination (including
boosters) underscore the need to continue evaluating repurposed drugs and
nutraceuticals as candidate adjunctive treatments. Early clinical studies of
antihistamines hypothesized that targeting mast cells (and/or histamine receptors)
might benefit COVID-19 patients. In cultured human coronary artery endothelial
cells, histamine potentiated spike-mediated angiotensin-converting enzyme 2
internalization; this effect can be blocked by the antihistamine famotidine. This
literature review focuses on clinical studies of antihistamines, mast cell stabilizers,
and leukotriene receptor antagonists for COVID-19 patients. Several antihistamines
and mast cell-targeting agents, including fluvoxamine, cyproheptadine, hydroxyzine,
and antihistamines used alone or with azithromycin (dexchlorpheniramine, cetirizine,
loratadine, and ebastine), as well as azelastine, famotidine (standard or high-dose),
high-dose famotidine with celecoxib, and the flavonoid mast cell stabilizer quercetin,
have been reported to be associated with clinical benefits in COVID-19 patients.
Multiple studies have reported mixed results for aspirin, montelukast, and normaldose famotidine; patients taking aspirin often have associated COVID-19 risk factors.
In the context of current standard-of-care treatments, clinical studies evaluating
candidate adjunctive treatments should carefully consider and avoid known
drug–drug interactions, such as those involving celecoxib and dexamethasone.
Further clinical studies of the identified treatments targeting mast cells and/or
histamine receptors in COVID-19 patients associated with clinical benefits are
therefore strongly recommended.
Keywords: COVID-19; Severe acute respiratory syndrome coronavirus 2; Mast cells;
Antihistamines; Famotidine; Quercetin
DOI record:
{
"DOI": "10.36922/imo025440058",
"ISSN": [
"3060-8910",
"3060-8740"
],
"URL": "http://dx.doi.org/10.36922/IMO025440058",
"abstract": "<jats:p>With the rollout of multiple COVID-19 vaccines, adjunctive treatments for COVID-19 have received less attention. Breakthrough infections post-vaccination (including boosters) underscore the need to continue evaluating repurposed drugs and nutraceuticals as candidate adjunctive treatments. Early clinical studies of antihistamines hypothesized that targeting mast cells (and/or histamine receptors) might benefit COVID-19 patients. In cultured human coronary artery endothelial cells, histamine potentiated spike-mediated angiotensin-converting enzyme 2 internalization; this effect can be blocked by the antihistamine famotidine. This literature review focuses on clinical studies of antihistamines, mast cell stabilizers, and leukotriene receptor antagonists for COVID-19 patients. Several antihistamines and mast cell-targeting agents, including fluvoxamine, cyproheptadine, hydroxyzine, and antihistamines used alone or with azithromycin (dexchlorpheniramine, cetirizine, loratadine, and ebastine), as well as azelastine, famotidine (standard or high-dose), high-dose famotidine with celecoxib, and the flavonoid mast cell stabilizer quercetin, have been reported to be associated with clinical benefits in COVID-19 patients. Multiple studies have reported mixed results for aspirin, montelukast, and normal-dose famotidine; patients taking aspirin often have associated COVID-19 risk factors. In the context of current standard-of-care treatments, clinical studies evaluating candidate adjunctive treatments should carefully consider and avoid known drug&ndash;drug interactions, such as those involving celecoxib and dexamethasone. Further clinical studies of the identified treatments targeting mast cells and/or histamine receptors in COVID-19 patients associated with clinical benefits are therefore strongly recommended.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Ricke",
"given": "Darrell O.",
"sequence": "first"
}
],
"container-title": "Innovative Medicines & Omics",
"container-title-short": "IMO",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
2,
5
]
],
"date-time": "2026-02-05T09:39:38Z",
"timestamp": 1770284378000
},
"deposited": {
"date-parts": [
[
2026,
2,
5
]
],
"date-time": "2026-02-05T09:39:38Z",
"timestamp": 1770284378000
},
"indexed": {
"date-parts": [
[
2026,
2,
5
]
],
"date-time": "2026-02-05T22:26:08Z",
"timestamp": 1770330368884,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "0",
"issued": {
"date-parts": [
[
2026,
2,
4
]
]
},
"journal-issue": {
"issue": "0",
"published-online": {
"date-parts": [
[
2026,
2,
5
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
2,
4
]
],
"date-time": "2026-02-04T00:00:00Z",
"timestamp": 1770163200000
}
}
],
"link": [
{
"URL": "https://api-journal.accscience.com/uploads/file/20260204/f50e3a40c1b325ac02d6906d1fb0f7d2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api-journal.accscience.com/uploads/file/20260204/f50e3a40c1b325ac02d6906d1fb0f7d2.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "22835",
"original-title": [],
"page": "025440058",
"prefix": "10.36922",
"published": {
"date-parts": [
[
2026,
2,
4
]
]
},
"published-online": {
"date-parts": [
[
2026,
2,
4
]
]
},
"publisher": "AccScience Publishing",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://accscience.com/journal/IMO/articles/online_first/6199"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Mast cells and histamine receptor-targeted adjunctive treatments for COVID-19: A literature review",
"type": "journal-article",
"volume": "0"
}
ricke