Association of Vitamin D with severity and outcome of COVID-19: Clinical and Experimental Evidence
et al., Journal of Innate Immunity, doi:10.1159/000535302, NCT04357366, Nov 2023
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 190 hospitalized COVID-19 patients showing vitamin D deficiency associated with increased disease severity and mortality. Authors also report on mouse experiments that show vitamin D reduced lung inflammation and downregulated inflammatory gene expression.
This is the 189th of 228 COVID-19 sufficiency studies for vitamin D, which collectively show higher levels reduce risk with p<0.0000000001.
|
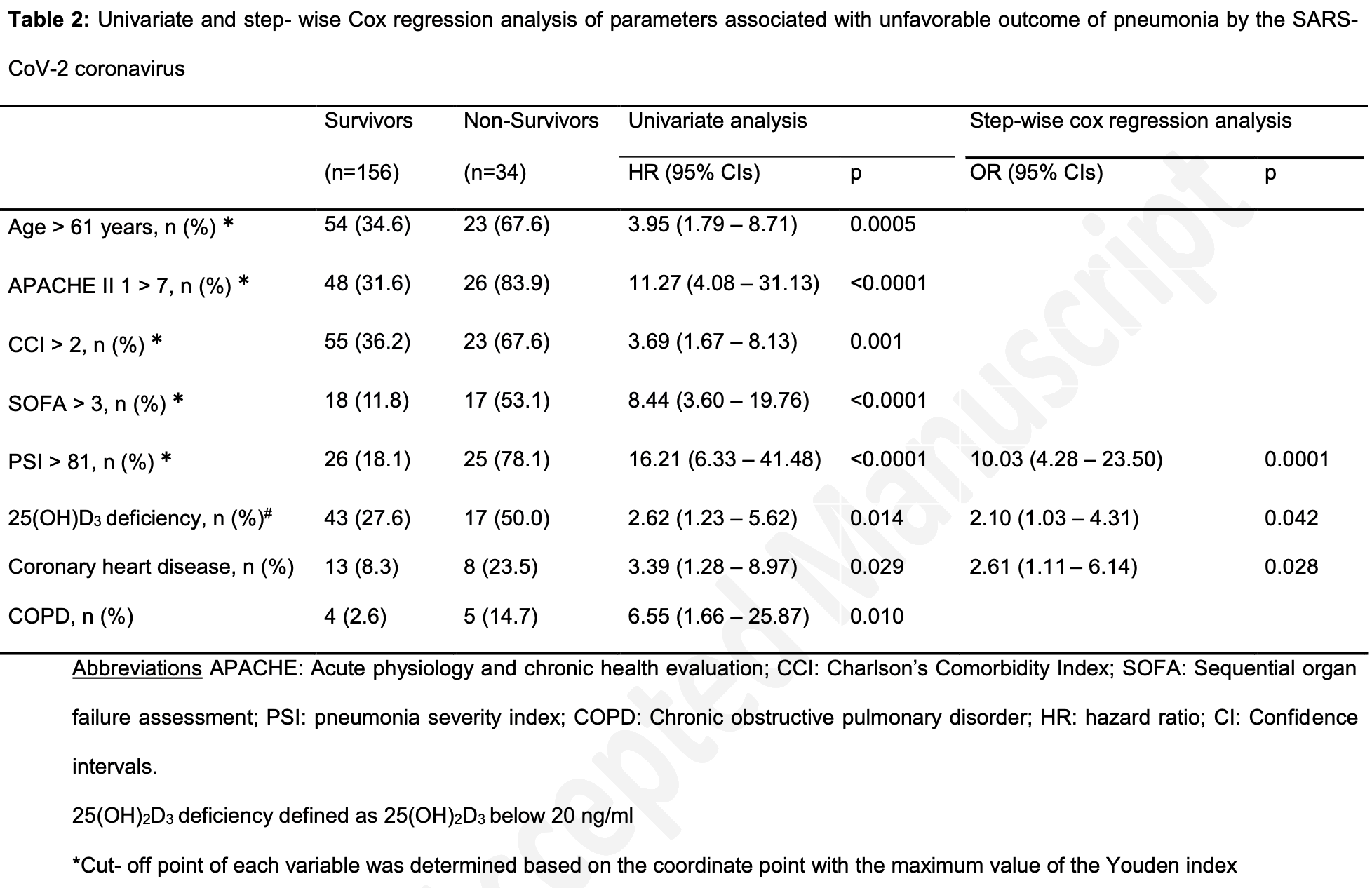
risk of death, 52.4% lower, HR 0.48, p = 0.04, high D levels (≥20ng/mL) 17 of 130 (13.1%), low D levels (<20ng/mL) 17 of 60 (28.3%), NNT 6.6, inverted to make HR<1 favor high D levels (≥20ng/mL).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Renieris et al., 26 Nov 2023, retrospective, Greece, peer-reviewed, 10 authors, trial NCT04357366 (history).
Contact: egiamarel@med.uoa.gr.
Association of Vitamin D with severity and outcome of COVID-19: Clinical and Experimental Evidence
Journal of Innate Immunity, doi:10.1159/000535302
Introduction: The role of vitamin in COVID-19 remains controversial. We investigated the association between endogenous vitamin D and the severity of COVID-19 as well as the mechanisms of action of vitamin D supplementation. Methods: 25(OH)D3 in serum was associated with disease severity and outcome in 190 COVID-19 patients. In a COVID-19 animal model using intravenous injection of plasma from patients with COVID-19 ARDS into C57/BL6 mice, mice were treated with 0.25μg human 1,25(OH)D3 or vehicle. Mice were sacrificed on day 4. Cytokines and myeloperoxidase (MPO) in tissues were measured. Changes in gene expression after vitamin D supplementation were measured. Results: Vitamin D deficiency and insufficiency were associated with increased severity and unfavourable outcome after 28 days. Vitamin D levels were negatively associated with biomarkers of COVID-19 severity. Vitamin D supplementation after challenge of mice with COVID-19 plasma led to reduced levels of TNFα, IL-6, IFNγ and MPO in the lung, as well as down-regulation of proinflammatory pathways. Conclusion: Normal levels of endogenous Vitamin D are associated with reduced severity and risk of unfavourable outcome in COVID-19, possibly through attenuation of tissue-specific hyperinflammation.
Statement of Ethics The SAVE trial (EudraCT number 2020-001466-11; ClinicalTrials.gov registration NCT04357366) was approved by the National Ethics Committee of Greece (approval 38/20) and by the National Organization for Medicines of Greece (approval ISO 28/20). The ESCAPE trial (EudraCT number 2020-001039-29; Clinicaltrials.gov NCT04339712) was approved by the National Ethics Committee of Greece (approval 30/20) and by the National Organization for Medicines of Greece (approval IS 021-20). Not mechanically ventilated patients were enrolled after written informed consent provided by themselves. Patients under mechanical ventilation were enrolled after written informed consent provided by their legal representative. Animal experiments were conducted in the Unit of Animals for Medical and Scientific purposes of the University General Hospital "Attikon" (Athens, Greece). All experiments were licensed from the Greek veterinary directorate under the protocol numbers 471955/06.07.2020 and 846137/07.07.2023.
Conflict of Interest Statement EJG-B has received honoraria from Abbott Products Operations AG, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH, Sobi and Xbiotech Inc; independent educational grants from Abbott CH, bioMérieux Inc, InflaRx GmbH, Johnson & Johnson, MSD, Sobi and UCB; and funding from the Horizon2020 Marie Skłodowska-Curie International Training Network "the European Sepsis Academy" (granted to the National and Kapodistrian University of..
References
Afgan, Baker, Van Den Beek, The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update, Nucleic Acids Res
Anders, Pyl, Huber, HTSeq--a Python framework to work with high-throughput sequencing data, Bioinformatics
Bishop, Ashfaq, Melnick, REsCue trial: Randomized controlled clinical trial with extended-release calcifediol in symptomatic COVID-19 outpatients, Nutrition
Bui, Zhu, Hawkins, Cortez-Resendiz, Bellon, Vitamin D regulation of the immune system and its implications for COVID-19: A mini review, SAGE Open Medicine
Cannell, Vieth, Umhau, Epidemic influenza and vitamin D, Epidemiol Infect
Chen, Mei, Xie, Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: a meta-analysis and GRADE assessment of cohort studies and RCTs, Nutr J
Crafa, Cannarella, Condorelli, Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: A systematic review and meta-analysis, EClinicalMedicine
Holick, Binkley, Bischoff-Ferrari, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline, J Clin Endocrinol Metab
Jolliffe, Griffiths, Martineau, Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies, The Journal of Steroid Biochemistry and Molecular Biology
Kim, Langmead, Salzberg, HISAT: a fast spliced aligner with low memory requirements, Nat Methods
Konikowska, Kiliś-Pstrusińska, Matera-Witkiewicz, Association of serum vitamin D concentration with the final course of hospitalization in patients with COVID-19, Front Immunol
Kuleshov, Jones, Rouillard, Enrichr: a comprehensive gene set enrichment analysis web server 2016 update, Nucleic Acids Res
Kyriazopoulou, Poulakou, Milionis, Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial, Nat Med
Lakkireddy, Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease, Scientific Reports
Love, Huber, Anders, Moderated estimation of fold change and dispersion for RNAseq data with DESeq2, Genome Biol
Maha, Can Vitamin D Deficiency Increase the Susceptibility to COVID-19?, Frontiers in Physiology
Meng, Li, Liu, The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials, Clinical Nutrition
Netea, Rovina, Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure, Cell Host & Microbe
Nogues, Ovejero, Pineda-Moncusí, Calcifediol treatment and COVID-19-related outcomes
Ramírez, Ryan, Grüning, deepTools2: a next generation web server for deepsequencing data analysis, Nucleic Acids Res
Renieris, Karakike, Gkavogianni, IL-1 Mediates Tissue Specific Inflammation and Severe Respiratory Failure In Covid-19: Clinical And Experimental Evidence, Infectious Diseases, doi:10.1101/2021.04.09.21255190
Robinson, Thorvaldsdóttir, Winckler, Integrative genomics viewer, Nat Biotechnol
Rodrigues, Sá, De, Ishimoto, Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, Journal of Experimental Medicine
Sassi, Tamone, Amelio, Vitamin D: Nutrient, Hormone, and Immunomodulator, Nutrients
Wang, Joshi, Leopold, Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis, Clinical Endocrinology
Wang, Wang, Li, RSeQC: quality control of RNA-seq experiments, Bioinformatics
Yisak, Ewunetei, Kefale, Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review, RMHP
Zhou, Wang, Yang, Treatment with 1,25(OH)2D3 induced HDAC2 expression and reduced NF-kB p65 expression in a rat model of OVA-induced asthma, Braz J Med Biol Res
DOI record:
{
"DOI": "10.1159/000535302",
"ISSN": [
"1662-811X",
"1662-8128"
],
"URL": "http://dx.doi.org/10.1159/000535302",
"abstract": "<jats:p>Introduction: The role of vitamin in COVID-19 remains controversial. We investigated the association between endogenous vitamin D and the severity of COVID-19 as well as the mechanisms of action of vitamin D supplementation.\nMethods: 25(OH)D3 in serum was associated with disease severity and outcome in 190 COVID-19 patients. In a COVID-19 animal model using intravenous injection of plasma from patients with COVID-19 ARDS into C57/BL6 mice, mice were treated with 0.25μg human 1,25(OH)D3 or vehicle. Mice were sacrificed on day 4. Cytokines and myeloperoxidase (MPO) in tissues were measured. Changes in gene expression after vitamin D supplementation were measured.\nResults: Vitamin D deficiency and insufficiency were associated with increased severity and unfavourable outcome after 28 days. Vitamin D levels were negatively associated with biomarkers of COVID-19 severity. Vitamin D supplementation after challenge of mice with COVID-19 plasma led to reduced levels of TNFα, IL-6, IFNγand MPO in the lung, as well as down-regulation of pro-inflammatory pathways.\nConclusion: Normal levels of endogenous Vitamin D are associated with reduced severity and risk of unfavourable outcome in COVID-19, possibly through attenuation of tissue-specific hyperinflammation. </jats:p>",
"author": [
{
"affiliation": [],
"family": "Renieris",
"given": "Georgios",
"sequence": "first"
},
{
"affiliation": [],
"family": "Foutadakis",
"given": "Spyros",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andriopoulou",
"given": "Theano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spanou",
"given": "Victoria-Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Droggiti",
"given": "Dionysia-Eirini",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kafousopoulos",
"given": "Dionysios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gkavogianni",
"given": "Theologia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Damoraki",
"given": "Georgia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vatsellas",
"given": "Giannis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giamarellos-Bourboulis",
"given": "Evangelos J.",
"sequence": "additional"
}
],
"container-title": "Journal of Innate Immunity",
"container-title-short": "J Innate Immun",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T22:00:32Z",
"timestamp": 1701122432000
},
"deposited": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T22:01:00Z",
"timestamp": 1701122460000
},
"indexed": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T00:52:00Z",
"timestamp": 1701132720001
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
26
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
26
]
],
"date-time": "2023-11-26T00:00:00Z",
"timestamp": 1700956800000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
26
]
],
"date-time": "2023-11-26T00:00:00Z",
"timestamp": 1700956800000
}
}
],
"link": [
{
"URL": "https://karger.com/jin/article-pdf/doi/10.1159/000535302/4051540/000535302.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://karger.com/jin/article-pdf/doi/10.1159/000535302/4051540/000535302.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "127",
"original-title": [],
"prefix": "10.1159",
"published": {
"date-parts": [
[
2023,
11,
26
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
26
]
]
},
"publisher": "S. Karger AG",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://karger.com/doi/10.1159/000535302"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology and Allergy"
],
"subtitle": [],
"title": "Association of Vitamin D with severity and outcome of COVID-19: Clinical and Experimental Evidence",
"type": "journal-article"
}
