An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients
et al., Scientific Reports, doi:10.1038/s41598-022-10370-1, CTRI/2021/05/033544, Apr 2022
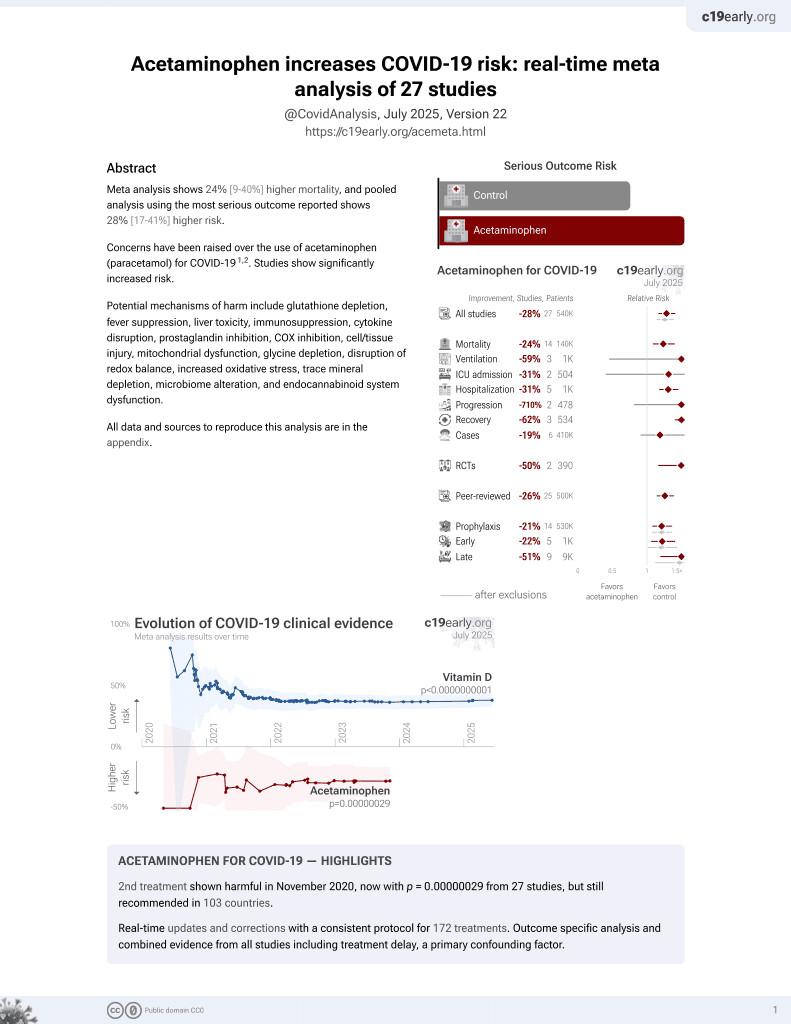
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
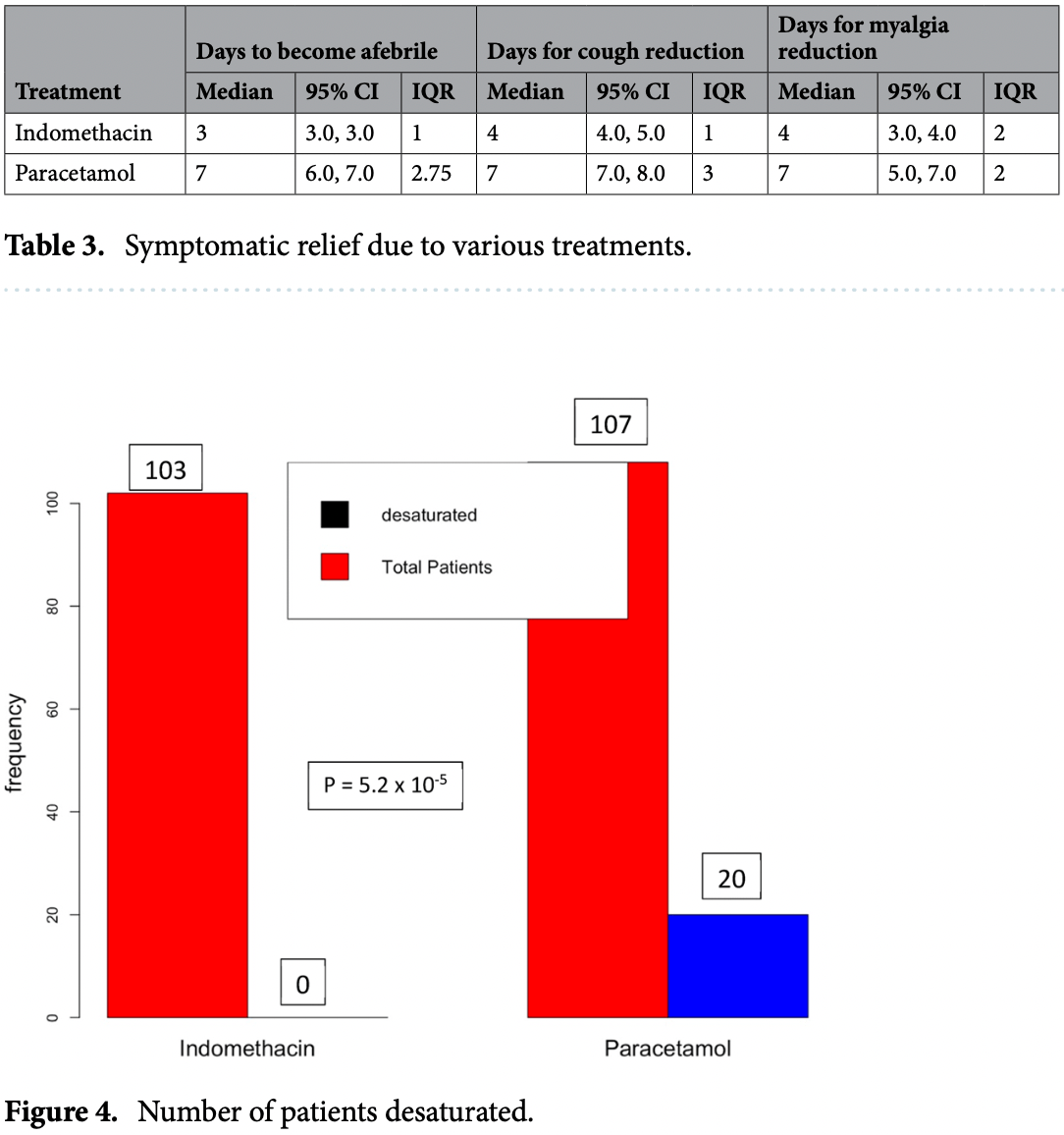
RCT with 107 paracetamol and 103 indomethacin patients, showing higher progression and worse recovery with paracetamol.
Paracetamol is also known as acetaminophen, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
Study covers acetaminophen and indomethacin.
|
risk of no recovery, 42.5% higher, RR 1.43, p = 0.002, treatment 77 of 107 (72.0%), control 52 of 103 (50.5%), day 14.
|
|
risk of progression, 3925.2% higher, RR 40.25, p < 0.001, treatment 20 of 107 (18.7%), control 0 of 103 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), SpO2 ≤93.
|
|
recovery time, 133.3% higher, relative time 2.33, p < 0.001, treatment median 7.0 IQR 2.75 n=107, control median 3.0 IQR 1.0 n=103, fever.
|
|
recovery time, 75.0% higher, relative time 1.75, p < 0.001, treatment median 7.0 IQR 2.0 n=107, control median 4.0 IQR 2.0 n=103, myalgia.
|
|
recovery time, 75.0% higher, relative time 1.75, p < 0.001, treatment median 7.0 IQR 3.0 n=107, control median 4.0 IQR 1.0 n=103, cough.
|
|
risk of no viral clearance, 20.1% higher, RR 1.20, p = 0.19, treatment 43 of 60 (71.7%), control 37 of 62 (59.7%), day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ravichandran et al., 19 Apr 2022, Randomized Controlled Trial, India, peer-reviewed, 8 authors, this trial compares with another treatment - results may be better when compared to placebo, trial CTRI/2021/05/033544.
Contact: rkkumar@iitm.ac.in.
An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients
Scientific Reports, doi:10.1038/s41598-022-10370-1
Indomethacin, a non-steroidal anti-inflammatory drug (NSAID), has been presented as a broadspectrum antiviral agent. This randomised clinical trial in a hospital setting evaluated the efficacy and safety of this drug in RT-PCR-positive coronavirus disease 2019 (COVID-19) patients. A total of 210 RT-PCR-positive COVID-19 patients who provided consent were allotted to the control or case arm, based on block randomisation. The control arm received standard of care comprising paracetamol, ivermectin, and other adjuvant therapies. The patients in the case arm received indomethacin instead of paracetamol, with other medications retained. The primary endpoint was the development of hypoxia/desaturation with SpO 2 ≤ 93, while time to become afebrile and time for cough and myalgia resolution were the secondary endpoints. The results of 210 patients were available, with 103 and 107 patients in the indomethacin and paracetamol arms, respectively. We monitored patient profiles along with everyday clinical parameters. In addition, blood chemistry at the time of admission and discharge was assessed. As no one in either of the arms required high-flow oxygen, desaturation with a SpO 2 level of 93 and below was the vital goal. In the indomethacin group, none of the 103 patients developed desaturation. On the other hand, 20 of the 107 patients in the paracetamol arm developed desaturation. Patients who received indomethacin also experienced more rapid symptomatic relief than those in the paracetamol arm, with most symptoms disappearing in half the time. In addition, 56 out of 107 in the paracetamol arm had fever on the seventh day, while no patient in the indomethacin group had fever. Neither arm reported any adverse event. The fourteenth-day follow-up revealed that the paracetamol arm patients had faced several discomforts; indomethacin arm patients mostly complained only of tiredness. Indomethacin is a safe and effective drug for treating patients with mild and moderate covid-19. SARS-Cov-2, a member of the coronavirus family, has been ravaging the world for the past 18 months. Although an effective treatment has eluded the medical community, there have been several registered trials on finding new or repurposed drugs. Several studies have discussed the mechanism of the virus-host interaction and possible treatments 1 , but safe and effective treatment for the disease is yet to emerge. Drug repurposing seems to be an immediate solution, and various drugs have been suggested for the COVID-19 treatment 2,3 . The drugs required to combat the pathogen may fall into one or more of the following categories: antivirals, anti-inflammatory agents, and supportive therapies 4,5 . According to V'Kovski 1 the antiviral action can be based
www.nature.com/scientificreports/ group was seven days. The median days for the resolution of cough and myalgia for the indomethacin group was four days, while it was seven days for the paracetamol group. We did not observe any adverse effects.
Supplementary data Anonymised patient data are given in supplementary data file 1. If further data is required, please contact the corresponding author.
Author contributions Author RR: Conception and lead resource and lead clinician. Authors KS, DK, SSD: Clinical study conduction, coordination and data collection. Author SS: Clinical help to A. Author SO: Test technician. Author SV: Protocol Design and data interpretation. Author KR: Data-analysis, interpretation, and manuscript preparation-Dr. Ramarathnam Krishna Kumar.
Competing interests The authors declare no competing interests.
References
Alkotaji, Al-Zidan, Indomethacin: can it counteract bradykinin effects in COVID-19 patients?, Curr. Pharmacol. Rep
Amici, Indomethacin has a potent antiviral activity against SARS coronavirus, Antivir. Ther
Amici, Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR, Cell. Microbiol
Bour, Westendorp, Laterveer, Bollen, Remarque, Interaction of indomethacin with cytokine production in whole blood. Potential mechanism for a brain-protective effect, Exp. Gerontol
Brunelli, The non-steroidal anti-inflammatory drug indomethacin activates the eIF2αkinase PKR, causing a translational block in human colorectal cancer cells, Biochem. J
Donnelly, Lloyd, Campbell, Indomethacin in rheumatoid arthritis: an evaluation of its anti-inflammatory and side effects, BMJ
First, Schroeder, Hariharan, Alexander, Weiskittel, The effect of indomethacin on the febrile response following OKT3 therapy1, Transplantation
Frediansyah, Tiwari, Sharun, Dhama, Harapan, Antivirals for COVID-19: a critical review, Clin. Epidemiol. Glob. Health
Gaughan, Francos, Dunn, Francos, Burke, A retrospective analysis of the effect of indomethacin on adverse reactions to orthoclone OKT3 in the therapy of acute renal allograft rejection, Am. J. Kidney Dis
Gil, COVID-19: drug targets and potential treatments, J. Med. Chem
Gomes, Cathepsin L in COVID-19: from pharmacological evidences to genetics, Front. Cell. Infect. Microbiol
Gordon, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Gordon, Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms, Science
Kanakaraj, Ravichandran, Low dose indomethacin in the outpatient treatment of COVID-19 in kidney transplant recipients-a case series, OALib
Liu, Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19, J. Clin. Virol
Mahmudpour, Roozbeh, Keshavarz, Farrokhi, Nabipour, COVID-19 cytokine storm: the anger of inflammation, Cytokine
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnol. Biotechnol. Equip
Napolitano, Gambardella, Carrella, Gao, Di Bernardo, Computational drug repositioning and elucidation of mechanism of action of compounds against SARS-CoV-2
Raghav, Kamboj, Singh, Effect of some steroidal and non-steroidal anti-inflammatory drugs on purified goat brain cathepsin L, Indian J. Med. Res. B Biomed. Res. Other Infect. Dis
Rajan, Subramanian, Clark, Low dose indomethacin for symptomatic treatment of COVID-19, Int. J. Med. Rev. Case Rep
Ravichandran, Use of indomethacin in COVID-19 patients: experience from two medical centres, J. Indian Med. Assoc
Saghaei, Random allocation software for parallel group randomized trials, BMC Med. Res. Methodol
Sakpal, Sample size estimation in clinical trial, Perspect. Clin. Res
Soy, Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment, Clin. Rheumatol
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: implications for SARS-CoV-2, Nat. Rev. Microbiol
Wang, Guan, COVID-19 drug repurposing: A review of computational screening methods, clinical trials, and protein interaction assays, Med. Res. Rev
Xu, Gao, Wu, Selinger, Zhou, Indomethacin has a potent antiviral activity against SARS CoV-2 in vitro and canine coronavirus in vivo, bioRxiv
Zhao, Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development, Signal Transd. Target. Therapy
DOI record:
{
"DOI": "10.1038/s41598-022-10370-1",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-10370-1",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Indomethacin, a non-steroidal anti-inflammatory drug (NSAID), has been presented as a broad-spectrum antiviral agent. This randomised clinical trial in a hospital setting evaluated the efficacy and safety of this drug in RT-PCR-positive coronavirus disease 2019 (COVID-19) patients. A total of 210 RT-PCR-positive COVID-19 patients who provided consent were allotted to the control or case arm, based on block randomisation. The control arm received standard of care comprising paracetamol, ivermectin, and other adjuvant therapies. The patients in the case arm received indomethacin instead of paracetamol, with other medications retained. The primary endpoint was the development of hypoxia/desaturation with SpO<jats:sub>2</jats:sub> ≤ 93, while time to become afebrile and time for cough and myalgia resolution were the secondary endpoints. The results of 210 patients were available, with 103 and 107 patients in the indomethacin and paracetamol arms, respectively. We monitored patient profiles along with everyday clinical parameters. In addition, blood chemistry at the time of admission and discharge was assessed. As no one in either of the arms required high-flow oxygen, desaturation with a SpO<jats:sub>2</jats:sub> level of 93 and below was the vital goal. In the indomethacin group, none of the 103 patients developed desaturation. On the other hand, 20 of the 107 patients in the paracetamol arm developed desaturation. Patients who received indomethacin also experienced more rapid symptomatic relief than those in the paracetamol arm, with most symptoms disappearing in half the time. In addition, 56 out of 107 in the paracetamol arm had fever on the seventh day, while no patient in the indomethacin group had fever. Neither arm reported any adverse event. The fourteenth-day follow-up revealed that the paracetamol arm patients had faced several discomforts; indomethacin arm patients mostly complained only of tiredness. Indomethacin is a safe and effective drug for treating patients with mild and moderate covid-19.\n</jats:p>",
"alternative-id": [
"10370"
],
"article-number": "6413",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "14 December 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "6 April 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "19 April 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Ravichandran",
"given": "Rajan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mohan",
"given": "Surapaneni Krishna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sukumaran",
"given": "Suresh Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamaraj",
"given": "Devakumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daivasuga",
"given": "Sumetha Suga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ravi",
"given": "Samson Oliver Abraham Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vijayaraghavalu",
"given": "Sivakumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Ramarathnam Krishna",
"sequence": "additional"
}
],
"container-title": [
"Scientific Reports"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T12:08:21Z",
"timestamp": 1650370101000
},
"deposited": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T12:09:09Z",
"timestamp": 1650370149000
},
"funder": [
{
"name": "Kris Gopalakrishnan"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T12:41:17Z",
"timestamp": 1650372077487
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2045-2322"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2022,
4,
19
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T00:00:00Z",
"timestamp": 1650326400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T00:00:00Z",
"timestamp": 1650326400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-10370-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-10370-1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-10370-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
4,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
19
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41579-020-00468-6",
"author": "P V’kovski",
"doi-asserted-by": "publisher",
"first-page": "155",
"issue": "3",
"journal-title": "Nat. Rev. Microbiol.",
"key": "10370_CR1",
"unstructured": "V’kovski, P., Kratzel, A., Steiner, S., Stalder, H. & Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19(3), 155–170 (2021).",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"author": "DE Gordon",
"doi-asserted-by": "publisher",
"first-page": "459",
"issue": "7816",
"journal-title": "Nature",
"key": "10370_CR2",
"unstructured": "Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583(7816), 459–468 (2020).",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1002/med.21728",
"author": "X Wang",
"doi-asserted-by": "publisher",
"first-page": "5",
"issue": "1",
"journal-title": "Med. Res. Rev.",
"key": "10370_CR3",
"unstructured": "Wang, X. & Guan, Y. COVID-19 drug repurposing: A review of computational screening methods, clinical trials, and protein interaction assays. Med. Res. Rev. 41(1), 5–28 (2021).",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00558-8",
"author": "M-M Zhao",
"doi-asserted-by": "publisher",
"first-page": "134",
"issue": "1",
"journal-title": "Signal Transd. Target. Therapy",
"key": "10370_CR4",
"unstructured": "Zhao, M.-M. et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transd. Target. Therapy 6(1), 134 (2021).",
"volume": "6",
"year": "2021"
},
{
"author": "CP Gomes",
"first-page": "10",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "10370_CR5",
"unstructured": "Gomes, C. P. et al. Cathepsin L in COVID-19: from pharmacological evidences to genetics. Front. Cell. Infect. Microbiol. 2020, 10 (2020).",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/j.cegh.2020.07.006",
"author": "A Frediansyah",
"doi-asserted-by": "publisher",
"first-page": "90",
"journal-title": "Clin. Epidemiol. Glob. Health",
"key": "10370_CR6",
"unstructured": "Frediansyah, A., Tiwari, R., Sharun, K., Dhama, K. & Harapan, H. Antivirals for COVID-19: a critical review. Clin. Epidemiol. Glob. Health 9, 90–98 (2021).",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1021/acs.jmedchem.0c00606",
"author": "C Gil",
"doi-asserted-by": "publisher",
"first-page": "12359",
"issue": "21",
"journal-title": "J. Med. Chem.",
"key": "10370_CR7",
"unstructured": "Gil, C. et al. COVID-19: drug targets and potential treatments. J. Med. Chem. 63(21), 12359–12386 (2020).",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1007/s10067-020-05190-5",
"author": "M Soy",
"doi-asserted-by": "publisher",
"first-page": "2085",
"issue": "7",
"journal-title": "Clin. Rheumatol.",
"key": "10370_CR8",
"unstructured": "Soy, M. et al. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 39(7), 2085–2094 (2020).",
"volume": "39",
"year": "2020"
},
{
"author": "DE Gordon",
"first-page": "1",
"issue": "10",
"journal-title": "Science",
"key": "10370_CR9",
"unstructured": "Gordon, D. E. et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science 9403(10), 1–38 (2020).",
"volume": "9403",
"year": "2020"
},
{
"DOI": "10.1177/135965350601100803",
"author": "C Amici",
"doi-asserted-by": "publisher",
"first-page": "1021",
"issue": "8",
"journal-title": "Antivir. Ther.",
"key": "10370_CR10",
"unstructured": "Amici, C. et al. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. 11(8), 1021–1030 (2006).",
"volume": "11",
"year": "2006"
},
{
"author": "T Xu",
"first-page": "8",
"journal-title": "bioRxiv",
"key": "10370_CR11",
"unstructured": "Xu, T., Gao, X., Wu, Z., Selinger, D. W. & Zhou, Z. Indomethacin has a potent antiviral activity against SARS CoV-2 in vitro and canine coronavirus in vivo. bioRxiv 12, 8 (2020).",
"volume": "12",
"year": "2020"
},
{
"key": "10370_CR12",
"unstructured": "Napolitano, F., Gambardella, G., Carrella, D., Gao, X., & di Bernardo, D. Computational drug repositioning and elucidation of mechanism of action of compounds against SARS-CoV-2 (2020)"
},
{
"author": "N Raghav",
"first-page": "188",
"journal-title": "Indian J. Med. Res. B Biomed. Res. Other Infect. Dis.",
"key": "10370_CR13",
"unstructured": "Raghav, N., Kamboj, R. C. & Singh, H. Effect of some steroidal and non-steroidal anti-inflammatory drugs on purified goat brain cathepsin L. Indian J. Med. Res. B Biomed. Res. Other Infect. Dis. 98, 188–192 (1993).",
"volume": "98",
"year": "1993"
},
{
"DOI": "10.1111/cmi.12446",
"author": "C Amici",
"doi-asserted-by": "publisher",
"first-page": "1391",
"issue": "9",
"journal-title": "Cell. Microbiol.",
"key": "10370_CR14",
"unstructured": "Amici, C. et al. Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR. Cell. Microbiol. 17(9), 1391–1404 (2015).",
"volume": "17",
"year": "2015"
},
{
"DOI": "10.1042/BJ20111236",
"author": "C Brunelli",
"doi-asserted-by": "publisher",
"first-page": "379",
"issue": "2",
"journal-title": "Biochem. J.",
"key": "10370_CR15",
"unstructured": "Brunelli, C. et al. The non-steroidal anti-inflammatory drug indomethacin activates the eIF2αkinase PKR, causing a translational block in human colorectal cancer cells. Biochem. J. 443(2), 379–386 (2012).",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1016/j.jcv.2020.104370",
"author": "F Liu",
"doi-asserted-by": "publisher",
"first-page": "104370",
"journal-title": "J. Clin. Virol.",
"key": "10370_CR16",
"unstructured": "Liu, F. et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 127, 104370 (2020).",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1016/j.cyto.2020.155151",
"author": "M Mahmudpour",
"doi-asserted-by": "publisher",
"first-page": "155151",
"journal-title": "Cytokine",
"key": "10370_CR17",
"unstructured": "Mahmudpour, M., Roozbeh, J., Keshavarz, M., Farrokhi, S. & Nabipour, I. COVID-19 cytokine storm: the anger of inflammation. Cytokine 133, 155151 (2020).",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.1016/S0531-5565(00)00128-5",
"author": "AMJ Bour",
"doi-asserted-by": "publisher",
"first-page": "1017",
"issue": "8",
"journal-title": "Exp. Gerontol.",
"key": "10370_CR18",
"unstructured": "Bour, A. M. J., Westendorp, R. G., Laterveer, J., Bollen, E. L. E. & Remarque, E. Interaction of indomethacin with cytokine production in whole blood. Potential mechanism for a brain-protective effect. Exp. Gerontol. 35(8), 1017–1024 (2000).",
"volume": "35",
"year": "2000"
},
{
"DOI": "10.1097/00007890-199201000-00017",
"author": "MR First",
"doi-asserted-by": "publisher",
"first-page": "91",
"issue": "1",
"journal-title": "Transplantation",
"key": "10370_CR19",
"unstructured": "First, M. R., Schroeder, T. J., Hariharan, S., Alexander, J. W. & Weiskittel, P. The effect of indomethacin on the febrile response following OKT3 therapy1. Transplantation 53(1), 91–93 (1992).",
"volume": "53",
"year": "1992"
},
{
"DOI": "10.1016/S0272-6386(12)80906-1",
"author": "WJ Gaughan",
"doi-asserted-by": "publisher",
"first-page": "486",
"issue": "3",
"journal-title": "Am. J. Kidney Dis.",
"key": "10370_CR20",
"unstructured": "Gaughan, W. J., Francos, B. B., Dunn, S. R., Francos, G. C. & Burke, J. F. A retrospective analysis of the effect of indomethacin on adverse reactions to orthoclone OKT3 in the therapy of acute renal allograft rejection. Am. J. Kidney Dis. 24(3), 486–490 (1994).",
"volume": "24",
"year": "1994"
},
{
"author": "R Ravichandran",
"first-page": "42",
"issue": "7",
"journal-title": "J. Indian Med. Assoc.",
"key": "10370_CR21",
"unstructured": "Ravichandran, R. et al. Use of indomethacin in COVID-19 patients: experience from two medical centres. J. Indian Med. Assoc. 119(7), 42–46 (2021).",
"volume": "119",
"year": "2021"
},
{
"DOI": "10.5455/IJMRCR.LOW-DOSE-INDOMETHACIN",
"author": "R Rajan",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Int. J. Med. Rev. Case Rep.",
"key": "10370_CR22",
"unstructured": "Rajan, R., Subramanian, S. & Clark, C. Low dose indomethacin for symptomatic treatment of COVID-19. Int. J. Med. Rev. Case Rep. 2020, 1 (2020).",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.4236/oalib.1106860",
"author": "A Kanakaraj",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "10",
"journal-title": "OALib.",
"key": "10370_CR23",
"unstructured": "Kanakaraj, A. & Ravichandran, R. Low dose indomethacin in the outpatient treatment of COVID-19 in kidney transplant recipients—a case series. OALib. 07(10), 1–8 (2020).",
"volume": "07",
"year": "2020"
},
{
"DOI": "10.1186/1471-2288-4-26",
"author": "M Saghaei",
"doi-asserted-by": "publisher",
"first-page": "26",
"issue": "1",
"journal-title": "BMC Med. Res. Methodol.",
"key": "10370_CR24",
"unstructured": "Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 4(1), 26 (2004).",
"volume": "4",
"year": "2004"
},
{
"author": "T Sakpal",
"first-page": "67",
"issue": "2",
"journal-title": "Perspect. Clin. Res.",
"key": "10370_CR25",
"unstructured": "Sakpal, T. Sample size estimation in clinical trial. Perspect. Clin. Res. 1(2), 67 (2010).",
"volume": "1",
"year": "2010"
},
{
"DOI": "10.1080/13102818.2020.1775118",
"author": "G Momekov",
"doi-asserted-by": "publisher",
"first-page": "469",
"issue": "1",
"journal-title": "Biotechnol. Biotechnol. Equip.",
"key": "10370_CR26",
"unstructured": "Momekov, G. & Momekova, D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens. Biotechnol. Biotechnol. Equip. 34(1), 469–474 (2020).",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1136/bmj.1.5532.69",
"author": "P Donnelly",
"doi-asserted-by": "publisher",
"first-page": "69",
"issue": "5532",
"journal-title": "BMJ",
"key": "10370_CR27",
"unstructured": "Donnelly, P., Lloyd, K. & Campbell, H. Indomethacin in rheumatoid arthritis: an evaluation of its anti-inflammatory and side effects. BMJ 1(5532), 69–75 (1967).",
"volume": "1",
"year": "1967"
},
{
"key": "10370_CR28",
"unstructured": "Indomethacin drug usage statistics, United States, 2018 [Internet]. 2018. https://clincalc.com/DrugStats/Drugs/Indomethacin"
},
{
"DOI": "10.1007/s40495-021-00257-6",
"author": "M Alkotaji",
"doi-asserted-by": "publisher",
"first-page": "102",
"issue": "3",
"journal-title": "Curr. Pharmacol. Rep.",
"key": "10370_CR29",
"unstructured": "Alkotaji, M. & Al-Zidan, R. N. Indomethacin: can it counteract bradykinin effects in COVID-19 patients?. Curr. Pharmacol. Rep. 7(3), 102–106 (2021).",
"volume": "7",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-10370-1"
}
},
"score": 1,
"short-container-title": [
"Sci Rep"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": [
"An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}
ravichandran