Efficacy of Kan Jang® in Patients with Mild COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial
et al., Pharmaceuticals, doi:10.3390/ph16091196, NCT04847518, Aug 2023
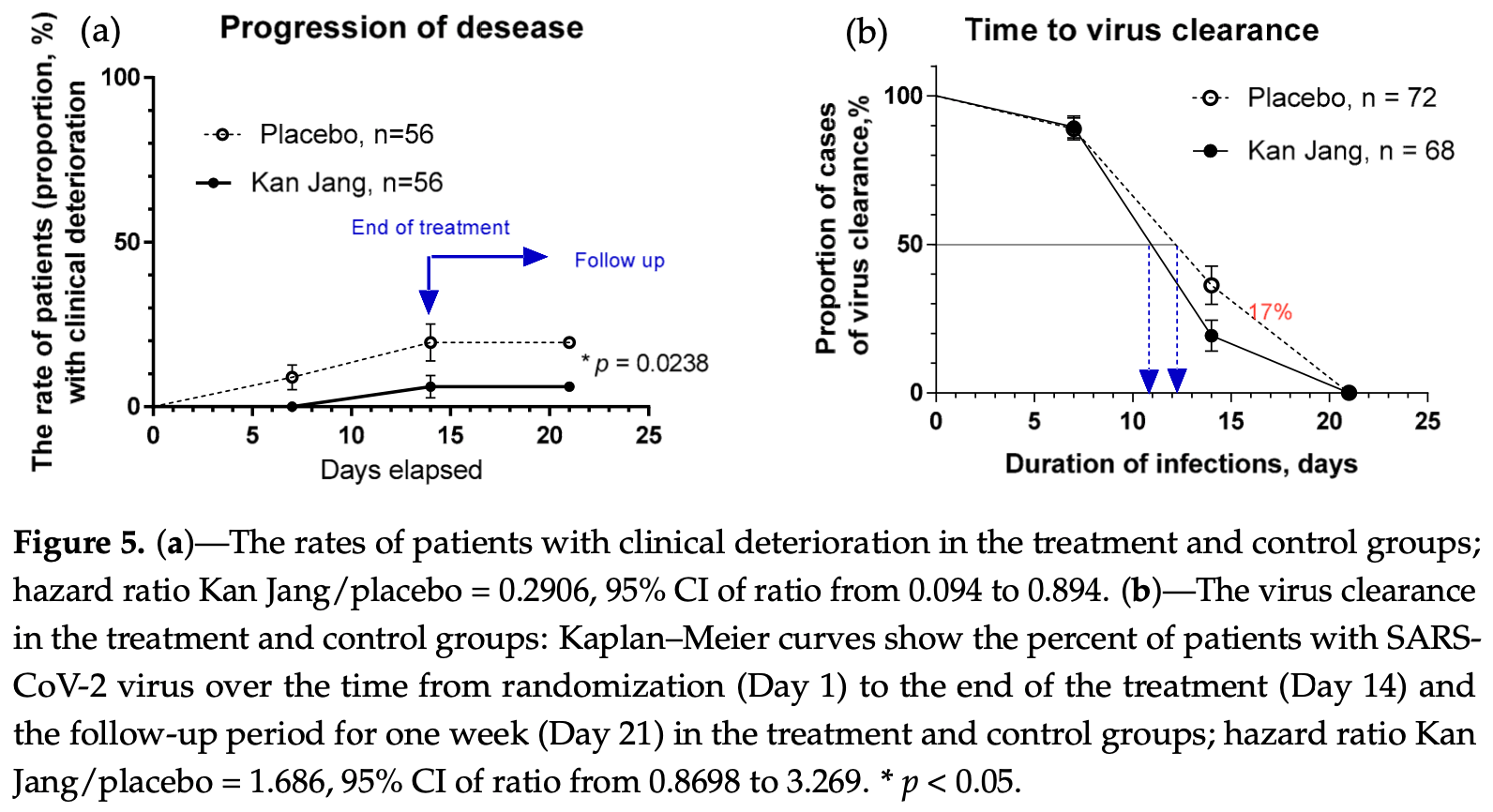
RCT 140 mild COVID-19 patients treated within 3 days of onset, showing lower progression and improved recovery with Kan Jang (andrographis + eleuthero).
|
risk of progression, 70.9% lower, HR 0.29, p = 0.03, treatment 3 of 56 (5.4%), control 10 of 56 (17.9%), NNT 8.0.
|
|
clinical effectiveness, 70.0% lower, RR 0.30, p = 0.07, treatment 3 of 56 (5.4%), control 10 of 56 (17.9%), NNT 8.0.
|
|
time to 50% clearance, 40.7% lower, HR 0.59, p = 0.12, treatment 56, control 56, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ratiani et al., 22 Aug 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Georgia, peer-reviewed, 6 authors, study period 26 May, 2021 - 30 October, 2022, this trial uses multiple treatments in the treatment arm (combined with Wall. ex. Nees and Eleutherococcus senticosus) - results of individual treatments may vary, trial NCT04847518 (history).
Efficacy of Kan Jang® in Patients with Mild COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial
Pharmaceuticals, doi:10.3390/ph16091196
Background and aim. This study aimed to assess the efficacy of the treatment of Kan Jang ® , a fixed combination of Andrographis paniculata (Burm. F.) Wall. ex. Nees and Eleutherococcus senticosus (Rupr. & Maxim.) Maxim extracts in patients with mild symptoms of COVID-19. Methods. One hundred and forty patients received six capsules of Kan Jang ® (n = 68, daily dose of andrographolides-90 mg) or placebo (n = 72) and supportive treatment (paracetamol) for 14 consecutive days in a randomized, quadruple-blinded, placebo-controlled, two-parallel-group design. The efficacy outcomes were the rate of cases turning to severe, the detection rate of coronavirus SARS-CoV-2 over the time of treatment, the duration, and the severity of symptoms (sore throat, runny nose, cough, headache, fatigue, loss of smell, taste, pain in muscles) in the acute phase of the disease. Other efficacy measures included improving cognitive and physical performance, quality of life, and the levels of inflammatory blood markers-interleukin 6 (IL-6), C-reactive protein, and D-dimer. Results. Kan Jang ® significantly (p < 0.05) reduced the rate of cases turning to severe (5.36%) compared to the placebo (17.86%) and decreased the detection rate of SARS-CoV-2 virus over the time of the treatment. The statistical difference in the rates of patients with clinical deterioration in the Kan Jang treatment and placebo control groups was significant (p = 0.0176) both in the 112 patients in the included-per-protocol (IPP) analysis and in the 140 patients in the intended-to-treat (ITT) analysis (p = 0.0236); the absolute risk reduction in cases thanks to the Kan Jang treatment was 12.5%, and the number we needed to treat with Kan Jang was 8. The patient's recovery time (number of sick days at the home/clinic) was shorter in the Kan Jang group compared with the placebo group. The rate of attenuation of inflammatory symptoms in the Kan Jang ® group was significantly higher, decreasing the severity of cough, sore throat/pain, runny nose, and muscle soreness compared with the placebo group. Kan Jang ® significantly decreased the Wisconsin Upper Respiratory Symptoms scores compared to the placebo in the sample size of 140 patients. However, the relief of fatigue and headache and the decrease in IL-6 in the blood were observed only in a subset of 86 patients infected during the second three waves of the pandemic. Kan Jang ® significantly increased physical activity and workout; however, it did not affect cognitive functions (attention and memory), quality of life score, inflammatory marker D-dimer, and C-reactive protein compared with the placebo group. Conclusions. Overall, the results of this study suggest that Kan Jang ® is effective in treating mild and moderate COVID-19 irrespective of the SARS-CoV-2 variant of infection.
Supplementary Materials: The following supporting information can be downloaded at: https:// www.mdpi.com/article/10.3390/ph16091196/s1. Informed Consent Statement: All participants provided written informed consent to join the study before inclusion.
Author Contributions:
Conflicts of Interest: The authors declare no conflict of interest. A.P. is self-employed at the research and development company Phytomed AB and has no shares or financial interest in any pharmaceutical company. The funders had no role in the study's design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
Azkur, Akdis, Azkur, Sokolowska, Van De Veen et al., Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19, Allergy, doi:10.1111/all.14364
Brekhman, On Antitoxic Action of Eleutherococcus
Gabrielian, Shukarian, Goukasova, Chandanian, Panossian et al., A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis, Phytomedicine, doi:10.1078/094471102321616391
Gagnier, Boon, Rochon, Moher, Barnes et al., Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration, J. Clin. Epidemiol, doi:10.1016/j.jclinepi.2005.12.020
Gupte, Hegde, Sawant, Kalathingal, Jadhav et al., Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: A retrospective analysis of active surveillance database, BMC Infect. Dis, doi:10.1186/s12879-021-07004-8
Intharuksa, Arunotayanun, Yooin, Sirisa-Ard, A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery, Molecules, doi:10.3390/molecules27144479
Izcovich, Siemieniuk, Bartoszko, Ge, Zeraatkar et al., Adverse effects of remdesivir, hydroxychloroquine and lopinavir/ritonavir when used for COVID-19: Systematic review and meta-analysis of randomised trials, BMJ Open, doi:10.1136/bmjopen-2020-048502
Jain, Iyengar, Vaishya, Differences between first wave and second wave of COVID-19 in India, Diabetol. Metab. Syndr, doi:10.1016/j.dsx.2021.05.009
Ji, Hu, Qiang, Lin, Pang et al., Traditional Chinese Medicine for COVID-19: A Network Meta-Analysis and Systematic Review, Am. J. Chin. Med, doi:10.1142/S0192415X22500379
Jiang, Xu, Zhou, Song, Liu et al., Contribution of traditional Chinese medicine combined with conventional western medicine treatment for the novel coronavirus disease (COVID-19), current evidence with systematic review and meta-analysis, Phytother. Res, doi:10.1002/ptr.7209
Kaewdech, Nawalerspanya, Assawasuwannakit, Chamroonkul, Jandee et al., The use of Andrographis paniculata and its effects on liver biochemistry of patients with gastrointestinal problems in Thailand during the COVID-19 pandemic: A cross sectional study, Sci. Rep, doi:10.1038/s41598-022-23189-7
Karbwang, Na-Bangchang, Repurposed drugs for COVID-19 treatment, J. Thai. Trad. Alt. Med
Kulichenko, Kireyeva, Malyshkina, Wikman, A randomized, controlled study of Kan Jang ® versus amantadine in the treatment of influenza in Volgograd, J. Herb. Pharmacother, doi:10.1080/J157v03n01_04
Lamb, Nirmatrelvir Plus Ritonavir: First Approval, Drugs, doi:10.1007/s40265-022-01692-5
Lega, Naviglio, Volpi, Tommasini, Recent Insight into SARS-CoV2 Immunopathology and Rationale for Potential Treatment and Preventive Strategies in COVID-19, doi:10.3390/vaccines8020224
Li, Fan, Lai, Han, Li et al., Coronavirus infections and immune responses, J. Med. Virol, doi:10.1002/jmv.25685
Liang, Fang, Liang, Lan, Shen et al., Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials, Complement. Ther. Med, doi:10.1016/j.ctim.2021.102744
Lim, Chan, Tan, Teh, Mohd Abd Razak et al., Andrographis paniculata (Burm. F.) Wall. Ex Nees, Andrographolide, and Andrographolide Analogues as SARS-CoV-2 Antivirals? A Rapid Review, doi:10.1177/1934578X211016610
Melchior, Palm, Wikman, Controlled clinical study of standardized Andrographis paniculata extract in common cold-A pilot trial, Phytomedicine, doi:10.1016/S0944-7113(97)80002-5
Melchior, Spasov, Ostrovskij, Bulanov, Wikman, Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan Jang ® ) in the treatment of uncomplicated upper-respiratory tract infection, Phytomedicine, doi:10.1016/S0944-7113(00)80053-7
Narimanyan, Jamalyan, Balyan, Barth, Palm et al., Early intervention with Kan Jang ® to treat upper-respiratory tract infections: A randomized, quadruple-blind study, J. Tradit. Complement. Med, doi:10.1016/j.jtcme.2021.06.001
Panossian, Brendler, The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections, Pharmaceuticals, doi:10.3390/ph13090236
Panossian, Efferth, Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity, Pharmaceuticals, doi:10.3390/ph15091051
Panossian, Seo, Klauck, Efferth, Adaptogens in chemobrain (part IV): Adaptogenic plants prevent the chemotherapeutics-induced imbalance of redox homeostasis by modulation of expression of genes encoding Nrf2-mediated signaling proteins and antioxidant, metabolizing, detoxifying enzymes in neuroglia cells, Longhua Chin. Med
Panossian, Seo, Wikman, Efferth, Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling, Phytomedicine, doi:10.1016/j.phymed.2015.08.004
Panossian, Wikman, Efficacy of Andrographis paniculata in upper respiratory tract (URT) infectious diseases and the mechanism of action
Ratiani, Pachkoria, Mamageishvili, Shengelia, Hovhannisyan et al., Efficacy of Kan Jang ® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial, Pharmaceuticals, doi:10.3390/ph15081013
Rattanaraksa, Khempetch, Poolwiwatchaikool, Nimitvilai, Loatrakul et al., The efficacy and safety of Andrographis paniculata extract for the treatment of COVID-19 patients with mild symptoms, Nakhonpathom hospital, Reg. Med. J
Schijns, Lavelle, Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity, Eur. J. Immunol, doi:10.1002/eji.202048693
Seo, Klauck, Efferth, Panossian, Adaptogens in chemobrain (Part II): Effect of plant extracts on chemotherapy induced cytotoxicity in neuroglia cells, Phytomedicine, doi:10.1016/j.phymed.2018.11.004
Seo, Klauck, Efferth, Panossian, Adaptogens in chemobrain (Part III): Antitoxic effects of plant extracts towards cancer chemotherapy-induced toxicity-transcriptome-wide microarray analysis of neuroglia cells, Phytomedicine
Shang, Shen, Stub, Zhu, Qiao et al., Adverse Effects of Andrographolide Derivative Medications Compared to the Safe use of Herbal Preparations of Andrographis paniculata: Results of a Systematic Review and Meta-Analysis of Clinical Studies, Front. Pharmacol, doi:10.3389/fphar.2022.773282
Shanker, Rangnekar, Wele, Soni, Gaikwad et al., A randomized controlled pilot study of add-on therapy of CIM-MEG19 (standardized Andrographis paniculata formulation) in mild to moderate COVID-19, doi:10.1016/j.phyplu.2022.100398
Spasov, Ostrovskij, Chernikov, Wikman, Comparative controlled study of Andrographis paniculata fixedcombination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children, Phytother. Res, doi:10.1002/ptr.1359
Tanwettiyanont, Piriyachananusorn, Sangsoi, Boonsong, Sunpapoa et al., The efficacy of Andrographis paniculata (Burm.f.) Wall. ex Nees crude extract in hospitalized mild COVID-19 patients: A retrospective cohort study, doi:10.1101/2022.01.01.22268609
Tanwettiyanont, Piriyachananusorn, Sangsoi, Boonsong, Sunpapoa et al., Use of Andrographis paniculata (Burm.f.) Wall. ex Nees and risk of pneumonia in hospitalised patients with mild coronavirus disease 2019: A retrospective cohort study, doi:10.3389/fmed.2022.947373
Tay, Poh, Rénia, Macary, Ng, The trinity of COVID-19: Immunity, inflammation and intervention, Nat. Rev. Immunol, doi:10.1038/s41577-020-0311-8
Tong, Ma, Zhou, Yang, Yang et al., Combination of Chinese herbal medicine and conventional western medicine for coronavirus disease 2019: A systematic review and meta-analysis, Front. Med, doi:10.3389/fmed.2023.1175827
Vardhana, Wolchok, The many faces of the anti-COVID immune response, J. Exp. Med, doi:10.1084/jem.20200678
Wanaratna, Leethong, Inchai, Chueawiang, Sriraksa et al., Efficacy and Safety of Andrographis paniculata Extract in Patients with Mild COVID-19: A Randomized Controlled Trial. medRxiv. 2021
Wu, Ji, Dai, Hei, Liang et al., Traditional Chinese medicine treatment for COVID-19: An overview of systematic reviews and meta-analyses, J. Integr. Med, doi:10.1016/j.joim.2022.06.006
Yang, Liu, Bai, Wang, Zhang et al., Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study, Pharmacol. Res, doi:10.1016/j.phrs.2020.104820
Yearsley, Thailand Approves Asian Herb Andrographis to Treat
Yuvejwattana, Thailand Clears Use of Herbal Medicine for COVID-19 Treatment; Bloomberg
Zhang, Lv, Zhou, Xie, Xu et al., Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial, Phytother. Res, doi:10.1002/ptr.7141
DOI record:
{
"DOI": "10.3390/ph16091196",
"ISSN": [
"1424-8247"
],
"URL": "http://dx.doi.org/10.3390/ph16091196",
"abstract": "<jats:p>Background and aim. This study aimed to assess the efficacy of the treatment of Kan Jang®, a fixed combination of Andrographis paniculata (Burm. F.) Wall. ex. Nees and Eleutherococcus senticosus (Rupr. & Maxim.) Maxim extracts in patients with mild symptoms of COVID-19. Methods. One hundred and forty patients received six capsules of Kan Jang® (n = 68, daily dose of andrographolides—90 mg) or placebo (n = 72) and supportive treatment (paracetamol) for 14 consecutive days in a randomized, quadruple-blinded, placebo-controlled, two-parallel-group design. The efficacy outcomes were the rate of cases turning to severe, the detection rate of coronavirus SARS-CoV-2 over the time of treatment, the duration, and the severity of symptoms (sore throat, runny nose, cough, headache, fatigue, loss of smell, taste, pain in muscles) in the acute phase of the disease. Other efficacy measures included improving cognitive and physical performance, quality of life, and the levels of inflammatory blood markers—interleukin 6 (IL-6), C-reactive protein, and D-dimer. Results. Kan Jang® significantly (p < 0.05) reduced the rate of cases turning to severe (5.36%) compared to the placebo (17.86%) and decreased the detection rate of SARS-CoV-2 virus over the time of the treatment. The statistical difference in the rates of patients with clinical deterioration in the Kan Jang treatment and placebo control groups was significant (p = 0.0176) both in the 112 patients in the included-per-protocol (IPP) analysis and in the 140 patients in the intended-to-treat (ITT) analysis (p = 0.0236); the absolute risk reduction in cases thanks to the Kan Jang treatment was 12.5%, and the number we needed to treat with Kan Jang was 8. The patient’s recovery time (number of sick days at the home/clinic) was shorter in the Kan Jang group compared with the placebo group. The rate of attenuation of inflammatory symptoms in the Kan Jang® group was significantly higher, decreasing the severity of cough, sore throat/pain, runny nose, and muscle soreness compared with the placebo group. Kan Jang® significantly decreased the Wisconsin Upper Respiratory Symptoms scores compared to the placebo in the sample size of 140 patients. However, the relief of fatigue and headache and the decrease in IL-6 in the blood were observed only in a subset of 86 patients infected during the second three waves of the pandemic. Kan Jang® significantly increased physical activity and workout; however, it did not affect cognitive functions (attention and memory), quality of life score, inflammatory marker D-dimer, and C-reactive protein compared with the placebo group. Conclusions. Overall, the results of this study suggest that Kan Jang® is effective in treating mild and moderate COVID-19 irrespective of the SARS-CoV-2 variant of infection.</jats:p>",
"alternative-id": [
"ph16091196"
],
"author": [
{
"affiliation": [
{
"name": "Department of Infectious Diseases, The First University Clinic, Tbilisi State Medical University, Gudamakari St., Tbilisi 0141, Georgia"
}
],
"family": "Ratiani",
"given": "Levan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, The First University Clinic, Tbilisi State Medical University, Gudamakari St., Tbilisi 0141, Georgia"
}
],
"family": "Pachkoria",
"given": "Elene",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department for History of Medicine and Bioethics, Faculty of Medicine, Tbilisi State Medical University, Vazha-Pshavela Ave. 33, Tbilisi 0162, Georgia"
}
],
"family": "Mamageishvili",
"given": "Nato",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department for History of Medicine and Bioethics, Faculty of Medicine, Tbilisi State Medical University, Vazha-Pshavela Ave. 33, Tbilisi 0162, Georgia"
}
],
"family": "Shengelia",
"given": "Ramaz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Fine Organic Chemistry of the National Academy of Science, Azatutian Ave. 26, Yerevan 375014, Armenia"
}
],
"family": "Hovhannisyan",
"given": "Areg",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8467-4525",
"affiliation": [
{
"name": "Phytomed AB, Sjöstadsvägen 6A, Lgh 1004, 59344 Västervik , Sweden"
}
],
"authenticated-orcid": false,
"family": "Panossian",
"given": "Alexander",
"sequence": "additional"
}
],
"container-title": "Pharmaceuticals",
"container-title-short": "Pharmaceuticals",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T12:58:54Z",
"timestamp": 1692709134000
},
"deposited": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T13:54:45Z",
"timestamp": 1692712485000
},
"funder": [
{
"award": [
"2021-1"
],
"name": "Swedish Herbal Institute AB in Vallberga, Sweden"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
23
]
],
"date-time": "2023-08-23T04:27:54Z",
"timestamp": 1692764874421
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2023,
8,
22
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2023,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T00:00:00Z",
"timestamp": 1692662400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1424-8247/16/9/1196/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1196",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
8,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
22
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1111/all.14364",
"article-title": "Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19",
"author": "Azkur",
"doi-asserted-by": "crossref",
"first-page": "1564",
"journal-title": "Allergy",
"key": "ref_1",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0311-8",
"article-title": "The trinity of COVID-19: Immunity, inflammation and intervention",
"author": "Tay",
"doi-asserted-by": "crossref",
"first-page": "363",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_2",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1084/jem.20200678",
"article-title": "The many faces of the anti-COVID immune response",
"author": "Vardhana",
"doi-asserted-by": "crossref",
"first-page": "e20200678",
"journal-title": "J. Exp. Med.",
"key": "ref_3",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25685",
"article-title": "Coronavirus infections and immune responses",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "424",
"journal-title": "J. Med. Virol.",
"key": "ref_4",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1002/eji.202048693",
"article-title": "Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity",
"author": "Schijns",
"doi-asserted-by": "crossref",
"first-page": "932",
"journal-title": "Eur. J. Immunol.",
"key": "ref_5",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.3390/vaccines8020224",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Lega, S., Naviglio, S., Volpi, S., and Tommasini, A. (2020). Recent Insight into SARS-CoV2 Immunopathology and Rationale for Potential Treatment and Preventive Strategies in COVID-19. Vaccines, 8."
},
{
"DOI": "10.1016/j.phrs.2020.104820",
"article-title": "Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "104820",
"journal-title": "Pharmacol. Res.",
"key": "ref_7",
"volume": "157",
"year": "2020"
},
{
"DOI": "10.3390/ph13090236",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Panossian, A., and Brendler, T. (2020). The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals, 13."
},
{
"DOI": "10.1016/j.phymed.2015.08.004",
"article-title": "Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling",
"author": "Panossian",
"doi-asserted-by": "crossref",
"first-page": "981",
"journal-title": "Phytomedicine",
"key": "ref_9",
"volume": "22",
"year": "2015"
},
{
"DOI": "10.3390/ph15091051",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Panossian, A., and Efferth, T. (2022). Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals, 15."
},
{
"DOI": "10.1016/S0944-7113(97)80002-5",
"article-title": "Controlled clinical study of standardized Andrographis paniculata extract in common cold—A pilot trial",
"author": "Melchior",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "Phytomedicine",
"key": "ref_11",
"volume": "3",
"year": "1997"
},
{
"DOI": "10.1016/S0944-7113(00)80053-7",
"article-title": "Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan Jang®) in the treatment of uncomplicated upper-respiratory tract infection",
"author": "Melchior",
"doi-asserted-by": "crossref",
"first-page": "341",
"journal-title": "Phytomedicine",
"key": "ref_12",
"volume": "7",
"year": "2000"
},
{
"DOI": "10.1078/094471102321616391",
"article-title": "A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis",
"author": "Gabrielian",
"doi-asserted-by": "crossref",
"first-page": "589",
"journal-title": "Phytomedicine",
"key": "ref_13",
"volume": "9",
"year": "2002"
},
{
"article-title": "A randomized, controlled study of Kan Jang® versus amantadine in the treatment of influenza in Volgograd",
"author": "Kulichenko",
"first-page": "188",
"journal-title": "J. Herb. Pharmacother.",
"key": "ref_14",
"volume": "173",
"year": "2003"
},
{
"DOI": "10.1002/ptr.1359",
"article-title": "Comparative controlled study of Andrographis paniculata fixedcombination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children",
"author": "Spasov",
"doi-asserted-by": "crossref",
"first-page": "47",
"journal-title": "Phytother. Res.",
"key": "ref_15",
"volume": "18",
"year": "2004"
},
{
"DOI": "10.1007/978-3-7091-0442-2",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Wagner, H., and Ulrich Merzenich, G. (2012). Evidence and Rational Based Research on Chinese Drugs, Springer."
},
{
"DOI": "10.1016/j.jtcme.2021.06.001",
"article-title": "Early intervention with Kan Jang® to treat upper-respiratory tract infections: A randomized, quadruple-blind study",
"author": "Narimanyan",
"doi-asserted-by": "crossref",
"first-page": "552",
"journal-title": "J. Tradit. Complement. Med.",
"key": "ref_17",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3390/ph15081013",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Ratiani, L., Pachkoria, E., Mamageishvili, N., Shengelia, R., Hovhannisyan, A., and Panossian, A. (2022). Efficacy of Kan Jang® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals, 15."
},
{
"DOI": "10.1016/j.dsx.2021.05.009",
"article-title": "Differences between first wave and second wave of COVID-19 in India",
"author": "Jain",
"doi-asserted-by": "crossref",
"first-page": "1047",
"journal-title": "Diabetol. Metab. Syndr.",
"key": "ref_19",
"volume": "15",
"year": "2021"
},
{
"key": "ref_20",
"unstructured": "World Health Organization (2023, July 28). Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance. Available online: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf."
},
{
"DOI": "10.1007/s40265-022-01692-5",
"article-title": "Nirmatrelvir Plus Ritonavir: First Approval",
"author": "Lamb",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Drugs",
"key": "ref_21",
"volume": "82",
"year": "2022"
},
{
"key": "ref_22",
"unstructured": "National Institutes of Health (2022, April 29). Antiviral Drugs That Are Approved, Authorized, or Under Evaluation for the Treatment of COVID-19, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf; pp. 140–221. https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_36.pdf."
},
{
"key": "ref_23",
"unstructured": "(2022, August 07). Molnupiravir_COVID19-MSD—Art 5(3)—Conditions for Use (europa.eu). Available online: https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-covid-19-article-53-procedure-conditions-use-conditions_en.pdf."
},
{
"key": "ref_24",
"unstructured": "Yuvejwattana, S. (2023, July 28). Thailand Clears Use of Herbal Medicine for COVID-19 Treatment; Bloomberg; 30 December 2020, 7:02 AM GMT+1. Available online: https://www.bloomberg.com/news/articles/2020-12-30/thailand-clears-use-of-herbal-medicine-for-covid-19-treatment."
},
{
"article-title": "Thailand Approves Asian Herb Andrographis to Treat COVID-19",
"author": "Yearsley",
"first-page": "35",
"journal-title": "HerbalGram.",
"key": "ref_25",
"volume": "129",
"year": "2021"
},
{
"article-title": "Repurposed drugs for COVID-19 treatment",
"author": "Karbwang",
"first-page": "285",
"journal-title": "J. Thai. Trad. Alt. Med.",
"key": "ref_26",
"volume": "19",
"year": "2021"
},
{
"key": "ref_27",
"unstructured": "World Health Organization (2022, August 07). Therapeutics and COVID-19. Therapeutic Management of Hospitalized Adults with COVID-19; Last Updated: 24 February 2022, Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinicalmanagement/hospitalized-adults--therapeutic-management/."
},
{
"DOI": "10.3389/fmed.2023.1175827",
"article-title": "Combination of Chinese herbal medicine and conventional western medicine for coronavirus disease 2019: A systematic review and meta-analysis",
"author": "Tong",
"doi-asserted-by": "crossref",
"first-page": "1175827",
"journal-title": "Front. Med.",
"key": "ref_28",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1142/S0192415X22500379",
"article-title": "Traditional Chinese Medicine for COVID-19: A Network Meta-Analysis and Systematic Review",
"author": "Ji",
"doi-asserted-by": "crossref",
"first-page": "883",
"journal-title": "Am. J. Chin. Med.",
"key": "ref_29",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1016/j.joim.2022.06.006",
"article-title": "Traditional Chinese medicine treatment for COVID-19: An overview of systematic reviews and meta-analyses",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "416",
"journal-title": "J. Integr. Med.",
"key": "ref_30",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7209",
"article-title": "Contribution of traditional Chinese medicine combined with conventional western medicine treatment for the novel coronavirus disease (COVID-19), current evidence with systematic review and meta-analysis",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "5992",
"journal-title": "Phytother. Res.",
"key": "ref_31",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.ctim.2021.102744",
"article-title": "Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "102744",
"journal-title": "Complement. Ther. Med.",
"key": "ref_32",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1101/2021.07.08.21259912",
"doi-asserted-by": "crossref",
"key": "ref_33",
"unstructured": "Wanaratna, K., Leethong, P., Inchai, N., Chueawiang, W., Sriraksa, P., Tabmee, A., and Sirinavin, S. (2022, August 07). Efficacy and Safety of Andrographis paniculata Extract in Patients with Mild COVID-19: A Randomized Controlled Trial. Available online: https://www.semanticscholar.org/paper/Efficacy-and-safety-of-Andrographis-paniculata-in-A-Wanaratna-Leethong/1f40a6181de4e0f15ab296673794210e82236455."
},
{
"article-title": "The efficacy and safety of Andrographis paniculata extract for the treatment of COVID-19 patients with mild symptoms, Nakhonpathom hospital",
"author": "Rattanaraksa",
"first-page": "269",
"journal-title": "Reg. Med. J.",
"key": "ref_34",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1101/2022.01.01.22268609",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Tanwettiyanont, J., Piriyachananusorn, N., Sangsoi, L., Boonsong, B., Sunpapoa, C., Tanamatayarat, P., Kanchanasurakit, S., and Na-Ek, N. (2022). The efficacy of Andrographis paniculata (Burm.f.) Wall. ex Nees crude extract in hospitalized mild COVID-19 patients: A retrospective cohort study. medRxiv."
},
{
"DOI": "10.1016/j.phyplu.2022.100398",
"article-title": "A randomized controlled pilot study of add-on therapy of CIM-MEG19 (standardized Andrographis paniculata formulation) in mild to moderate COVID-19",
"author": "Shanker",
"doi-asserted-by": "crossref",
"first-page": "100398",
"journal-title": "Phytomed. Plus.",
"key": "ref_36",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1002/ptr.7141",
"article-title": "Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "4401",
"journal-title": "Phytother. Res.",
"key": "ref_37",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-23189-7",
"article-title": "The use of Andrographis paniculata and its effects on liver biochemistry of patients with gastrointestinal problems in Thailand during the COVID-19 pandemic: A cross sectional study",
"author": "Kaewdech",
"doi-asserted-by": "crossref",
"first-page": "18213",
"journal-title": "Sci. Rep.",
"key": "ref_38",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2022.947373",
"article-title": "Use of Andrographis paniculata (Burm.f.) Wall. ex Nees and risk of pneumonia in hospitalised patients with mild coronavirus disease 2019: A retrospective cohort study",
"author": "Tanwettiyanont",
"doi-asserted-by": "crossref",
"first-page": "947373",
"journal-title": "Front. Med.",
"key": "ref_39",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.3390/molecules27144479",
"doi-asserted-by": "crossref",
"key": "ref_40",
"unstructured": "Intharuksa, A., Arunotayanun, W., Yooin, W., and Sirisa-Ard, P. (2022). A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules, 27."
},
{
"DOI": "10.1136/bmjopen-2020-048502",
"article-title": "Adverse effects of remdesivir, hydroxychloroquine and lopinavir/ritonavir when used for COVID-19: Systematic review and meta-analysis of randomised trials",
"author": "Izcovich",
"doi-asserted-by": "crossref",
"first-page": "e048502",
"journal-title": "BMJ Open",
"key": "ref_41",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1186/s12879-021-07004-8",
"doi-asserted-by": "crossref",
"key": "ref_42",
"unstructured": "Gupte, V., Hegde, R., Sawant, S., Kalathingal, K., Jadhav, S., Malabade, R., and Gogtay, J. (2022). Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: A retrospective analysis of active surveillance database. BMC Infect. Dis., 22."
},
{
"DOI": "10.3389/fphar.2022.773282",
"article-title": "Adverse Effects of Andrographolide Derivative Medications Compared to the Safe use of Herbal Preparations of Andrographis paniculata: Results of a Systematic Review and Meta-Analysis of Clinical Studies",
"author": "Shang",
"doi-asserted-by": "crossref",
"first-page": "773282",
"journal-title": "Front. Pharmacol.",
"key": "ref_43",
"volume": "13",
"year": "2022"
},
{
"key": "ref_44",
"unstructured": "Brekhman, I.I. (1982). On Antitoxic Action of Eleutherococcus, Meditsina."
},
{
"key": "ref_45",
"unstructured": "HMPC—Committee on HerbalMedicinal Products (2022, May 30). Assessment Report on Eleutherococcus senticosus (Rupr. etMaxim.) Maxim, Radix.EMA/HMPC/680615/2013. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-eleutherococcus-senticosus-rupr-et-maxim-maxim-radix_en.pdf."
},
{
"DOI": "10.1016/j.phymed.2018.11.004",
"article-title": "Adaptogens in chemobrain (Part II): Effect of plant extracts on chemotherapy induced cytotoxicity in neuroglia cells",
"author": "Seo",
"doi-asserted-by": "crossref",
"first-page": "152743",
"journal-title": "Phytomedicine",
"key": "ref_46",
"volume": "58",
"year": "2019"
},
{
"DOI": "10.1016/j.phymed.2018.11.011",
"article-title": "Adaptogens in chemobrain (Part III): Antitoxic effects of plant extracts towards cancer chemotherapy-induced toxicity-transcriptome-wide microarray analysis of neuroglia cells",
"author": "Seo",
"doi-asserted-by": "crossref",
"first-page": "246",
"journal-title": "Phytomedicine",
"key": "ref_47",
"volume": "56",
"year": "2019"
},
{
"DOI": "10.21037/lcm-20-24",
"article-title": "Adaptogens in chemobrain (part IV): Adaptogenic plants prevent the chemotherapeutics-induced imbalance of redox homeostasis by modulation of expression of genes encoding Nrf2-mediated signaling proteins and antioxidant, metabolizing, detoxifying enzymes in neuroglia cells",
"author": "Panossian",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Longhua Chin. Med.",
"key": "ref_48",
"volume": "3",
"year": "2020"
},
{
"article-title": "Andrographis paniculata (Burm. F.) Wall. Ex Nees, Andrographolide, and Andrographolide Analogues as SARS-CoV-2 Antivirals? A Rapid Review",
"author": "Lim",
"first-page": "1934578X211016610",
"journal-title": "Nat. Prod. Commun.",
"key": "ref_49",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/j.jclinepi.2005.12.020",
"article-title": "Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration",
"author": "Gagnier",
"doi-asserted-by": "crossref",
"first-page": "1134",
"journal-title": "J. Clin. Epidemiol.",
"key": "ref_50",
"volume": "59",
"year": "2006"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1424-8247/16/9/1196"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Drug Discovery",
"Pharmaceutical Science",
"Molecular Medicine"
],
"subtitle": [],
"title": "Efficacy of Kan Jang® in Patients with Mild COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial",
"type": "journal-article",
"volume": "16"
}