Effects of mega dose vitamin C in critically ill COVID-19 patients: a randomized control trial
et al., Biological and Clinical Sciences Research Journal, doi:10.54112/bcsrj.v2023i1.343, NCT04682574, Jun 2023
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
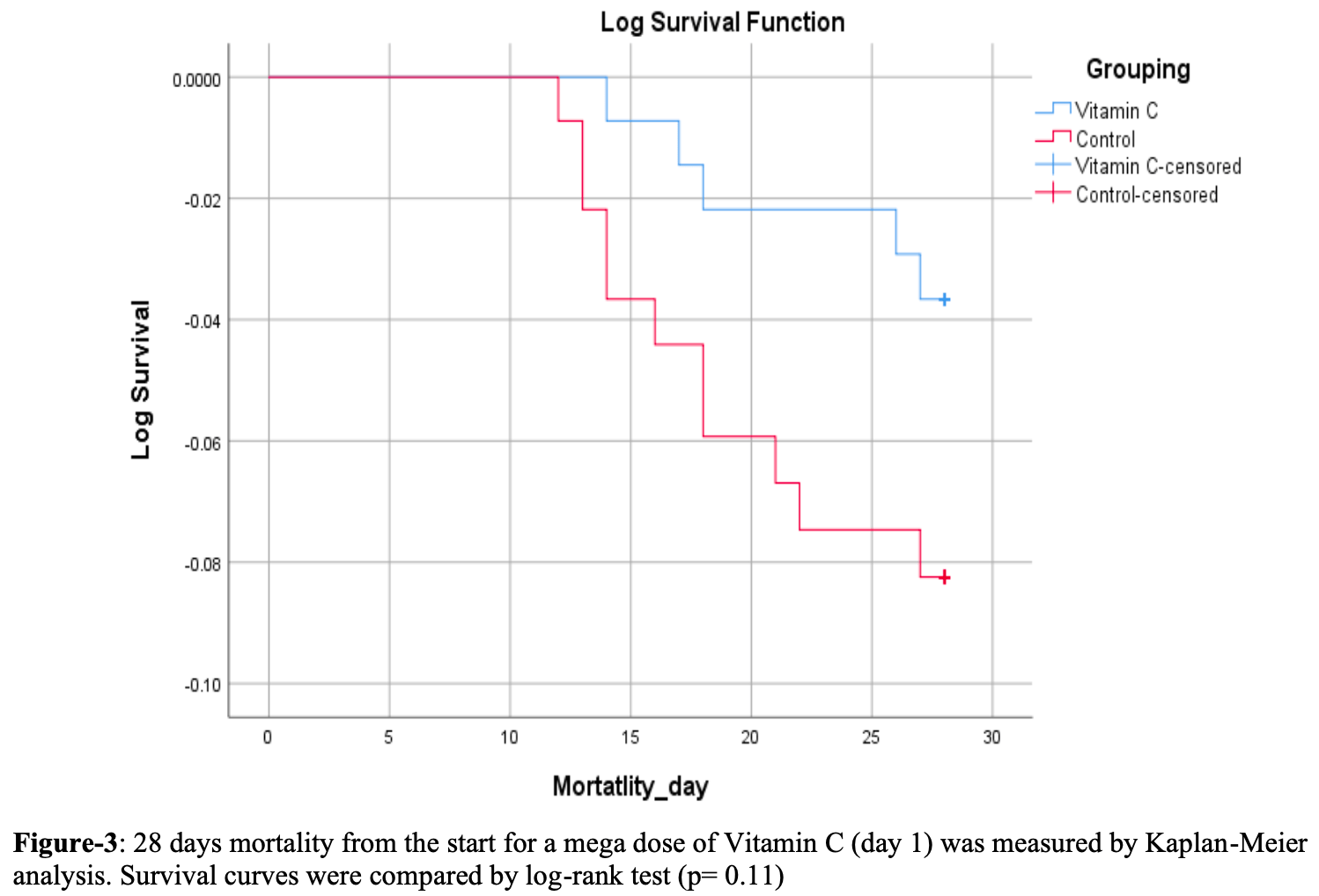
RCT 278 COVID-19 ICU patients in Pakistan, showing lower mortality and ventilation, and shorter length of stay with high-dose vitamin C treatment, without statistical significance. 30 grams IV vitamin C for four days.
This is the 16th of 21 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0012.
This is the 62nd of 74 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000068.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
risk of death, 54.5% lower, RR 0.45, p = 0.20, treatment 5 of 139 (3.6%), control 11 of 139 (7.9%), NNT 23.
|
|
risk of mechanical ventilation, 44.4% lower, RR 0.56, p = 0.41, treatment 5 of 139 (3.6%), control 9 of 139 (6.5%), NNT 35.
|
|
hospitalization time, 36.8% lower, relative time 0.63, p = 0.91, treatment 139, control 139.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rana et al., 28 Jun 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Pakistan, peer-reviewed, 10 authors, study period 28 December, 2020 - 10 April, 2022, dosage 30000mg days 1-4, trial NCT04682574 (history).
Contact: mansoorhafeez140@outlook.com.
EFFECTS OF MEGA DOSE VITAMIN C IN CRITICALLY ILL COVID-19 PATIENTS: A RANDOMIZED CONTROL TRIAL
Biological and Clinical Sciences Research Journal, doi:10.54112/bcsrj.v2023i1.343
Severe acute respiratory coronavirus 2 (SARS-CoV-2), COVID-19, caused a pandemic that took millions of lives worldwide. The main reason is a lack of preparedness and knowledge about the treatment options. With the advancement in the understanding of the SARS-CoV-2 virus, many treatment options have been analyzed that helped effectively to decrease the mortality caused by this virus. Vitamin C is known to boost immunity and slow down the progression of viral infection. The current study was designed to assess the effectiveness of a high dose of intravenous (IV) vitamin C in COVID-19 infection. The clinical trial registered on 23/12/2020 at ClinicalTrials.gov (NCT04682574) was conducted in Bahria Town International Hospital, Lahore (BTIHL), Fatima Memorial Hospital, Lahore (FMH), and Evercare Hospital Lahore from 28/12/2020 to 10/4/2022. Two hundred seventy-eight critically ill patients with COVID-19 were categorized into two groups. One hundred thirty-nine patients were randomized in group VC (vitamin C), which was given a high dose (30 grams) of intravenous (IV) vitamin C for four days, whereas distilled water as a placebo was given to the control group (n=139) along with standard treatment protocols. All the patients were analyzed for primary outcomes in partial pressure of arterial oxygen (PaO2) to Fraction inspired oxygen (P/F) ratio and survival analysis. At the same time, levels of inflammatory and biochemical markers needed for intubation and length of hospital stay in both groups were compared as the study's secondary endpoint. Among the two groups, we did not find any differences in 28-day mortality (Log Rank P= 0.11). Similarly, no difference in the P/F ratio on the fourth day after the start of IV vitamin C treatment was noted (p=0.24). The median values of biochemical and inflammatory variables improved significantly in group VC on day 4. However, only hemoglobin levels remained non-significant between the groups. Mean days of hospital stay were slightly longer in group C. However, no statistical significance (p=0.941) was found. Although Group VC needed fewer intubations than Group C, results remained statistically insignificant (p= 0.273). This trial did not find any mortality benefit or improvement of the P/F ratio in critically ill patients. However, the VC group showed improvement in biochemical variables of prognostic importance, which seems to lower the chance of intubation and LOS in group VC. A further clinical trial with a large sample size is needed to reach the final conclusion.
Conflict of interest The authors declared an absence of conflict of interest.
References
Abobaker, Alzwi, Alraied, Overview of the possible role of vitamin C in management of COVID-19, Pharmacological Reports
Bartleson, Radenkovic, Covarrubias, Furman, Winer et al., Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome, PharmaNutrition
Calder, Carr, Gombart, Eggersdorfer, Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections, Nutrients
Chen, Dong, Lei, Li, Gao et al., Vitamin C suppresses toxicological effects in MO/MФ and IgM+ B cells of Nile tilapia (Oreochromis niloticus) upon copper exposure, Aquatic Toxicology
Cheng, Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)?, Medicine in drug discovery
Chu, Tang, Huang, Hao, Wei, Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264. 7 macrophages by suppressing MAPK and NF-κb signal pathways, Environmental toxicology and pharmacology
Faqihi, Alharthy, Alodat, Kutsogiannis, Brindley et al., Therapeutic plasma exchange in adult critically ill patients with lifethreatening SARS-CoV-2 disease: a pilot study, Journal of critical care
Goud, Bai, Abu-Soud, A multiple-hit hypothesis involving reactive oxygen species and myeloperoxidase explains clinical deterioration and fatality in COVID-19, International Journal of Biological Sciences
Hemilä, Carr, Chalker, Vitamin C may increase the recovery rate of outpatient cases of SARS-CoV-2 infection by 70%: reanalysis of the COVID A to Z randomized clinical trial, Frontiers in immunology
Hemilä, Chalker, Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a metaregression analysis, Journal of intensive care
Islam, Quispe, Martorell, Docea, Salehi et al., Dietary supplements, vitamins and minerals as potential interventions against viruses: Perspectives for COVID-19, International Journal for Vitamin and Nutrition Research
Jafari, Esmaeilzadeh, Mohammadi-Kordkhayli, Rezaei, Vitamin C and the immune system
Jain, Chaurasia, Sengar, Singh, Mahor et al., Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers, Scientific reports
Jovic, Ali, Ibrahim, Jessop, Tarassoli et al., Could vitamins help in the fight against COVID-19?, Nutrients
Lai, Shih, Ko, Tang, Hsueh, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges, International journal of antimicrobial agents
Marazuela, Giustina, Puig-Domingo, Matthay, Zemans et al., Effects of mega dose vitamin c in critically ill covid-19 patients: a randomized control trial, doi:10.54112/bcsrj.v2023i1.282
Ran, Zhao, Wang, Zhao, Bu, Vitamin C as a supplementary therapy in relieving symptoms of the common cold: a meta-analysis of 10 randomized controlled trials, BioMed Research International
Siordia, Bernaba, Yoshino, Ulhaque, Kumar et al., Systematic and statistical review of coronavirus disease 19 treatment trials, SN comprehensive clinical medicine
Song, Zhang, Yin, Wang, Zhou et al., COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2), International journal of antimicrobial agents
Talukdar, Bhadra, Dattaroy, Nagle, Dasgupta, Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19, Biomedicine & Pharmacotherapy
Vorilhon, Arpajou, Vaillant Roussel, Merlin, Pereira et al., Efficacy of vitamin C for the prevention and treatment of upper respiratory tract infection. A meta-analysis in children, European journal of clinical pharmacology
Yaghoubi, Youssefi, Jabbari Azad, Farzad, Yavari et al., Total antioxidant capacity as a marker of severity of COVID-19 infection: Possible prognostic and therapeutic clinical application, Journal of Medical Virology
Yoo, Kim, Ju, Lee, Cho et al., Clinical impact of supplementation of vitamins B1 and C on patients with sepsisrelated acute respiratory distress syndrome. Tuberculosis and Respiratory Diseases 83, 248, Annals of intensive care
DOI record:
{
"DOI": "10.54112/bcsrj.v2023i1.343",
"ISSN": [
"2708-2261",
"2958-4728"
],
"URL": "http://dx.doi.org/10.54112/bcsrj.v2023i1.343",
"abstract": "<jats:p>Severe acute respiratory coronavirus 2 (SARS-CoV-2), COVID-19, caused a pandemic that took millions of lives worldwide. The main reason is a lack of preparedness and knowledge about the treatment options. With the advancement in the understanding of the SARS-CoV-2 virus, many treatment options have been analyzed that helped effectively to decrease the mortality caused by this virus. Vitamin C is known to boost immunity and slow down the progression of viral infection. The current study was designed to assess the effectiveness of a high dose of intravenous (IV) vitamin C in COVID-19 infection. The clinical trial registered on 23/12/2020 at ClinicalTrials.gov (NCT04682574) was conducted in Bahria Town International Hospital, Lahore (BTIHL), Fatima Memorial Hospital, Lahore (FMH), and Evercare Hospital Lahore from 28/12/2020 to 10/4/2022. Two hundred seventy-eight critically ill patients with COVID-19 were categorized into two groups. One hundred thirty-nine patients were randomized in group VC (vitamin C), which was given a high dose (30 grams) of intravenous (IV) vitamin C for four days, whereas distilled water as a placebo was given to the control group (n=139) along with standard treatment protocols. All the patients were analyzed for primary outcomes in partial pressure of arterial oxygen (PaO2) to Fraction inspired oxygen (P/F) ratio and survival analysis. At the same time, levels of inflammatory and biochemical markers needed for intubation and length of hospital stay in both groups were compared as the study's secondary endpoint. Among the two groups, we did not find any differences in 28-day mortality (Log Rank P= 0.11). Similarly, no difference in the P/F ratio on the fourth day after the start of IV vitamin C treatment was noted (p=0.24). The median values of biochemical and inflammatory variables improved significantly in group VC on day 4. However, only hemoglobin levels remained non-significant between the groups. Mean days of hospital stay were slightly longer in group C. However, no statistical significance (p=0.941) was found. Although Group VC needed fewer intubations than Group C, results remained statistically insignificant (p= 0.273). This trial did not find any mortality benefit or improvement of the P/F ratio in critically ill patients. However, the VC group showed improvement in biochemical variables of prognostic importance, which seems to lower the chance of intubation and LOS in group VC. A further clinical trial with a large sample size is needed to reach the final conclusion.</jats:p>",
"author": [
{
"affiliation": [],
"family": "RANA",
"given": "MA",
"sequence": "first"
},
{
"affiliation": [],
"family": "IQBAL",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "CHAUDHRY",
"given": "KA",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HAFEEZ",
"given": "MM",
"sequence": "additional"
},
{
"affiliation": [],
"family": "JAVED",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "IQBAL",
"given": "W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HASHMI",
"given": "MS",
"sequence": "additional"
},
{
"affiliation": [],
"family": "PERVAIZ",
"given": "R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "SIDDIQUI",
"given": "MH",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AWAD",
"given": "AHA",
"sequence": "additional"
}
],
"container-title": "Biological and Clinical Sciences Research Journal",
"container-title-short": "Biol Clin Sci Res J",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
13
]
],
"date-time": "2023-07-13T04:13:01Z",
"timestamp": 1689221581000
},
"deposited": {
"date-parts": [
[
2023,
7,
14
]
],
"date-time": "2023-07-14T04:13:24Z",
"timestamp": 1689308004000
},
"indexed": {
"date-parts": [
[
2023,
7,
15
]
],
"date-time": "2023-07-15T04:21:38Z",
"timestamp": 1689394898464
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
6,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
1,
9
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
28
]
],
"date-time": "2023-06-28T00:00:00Z",
"timestamp": 1687910400000
}
}
],
"link": [
{
"URL": "http://bcsrj.com/ojs/index.php/bcsrj/article/download/343/456",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://bcsrj.com/ojs/index.php/bcsrj/article/download/343/456",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "32320",
"original-title": [],
"page": "343",
"prefix": "10.54112",
"published": {
"date-parts": [
[
2023,
6,
28
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
28
]
]
},
"publisher": "Medeye Publishers",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://bcsrj.com/ojs/index.php/bcsrj/article/view/343"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "EFFECTS OF MEGA DOSE VITAMIN C IN CRITICALLY ILL COVID-19 PATIENTS: A RANDOMIZED CONTROL TRIAL",
"type": "journal-article",
"volume": "2023"
}
