A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.100957, NCT04311177, Jul 2021
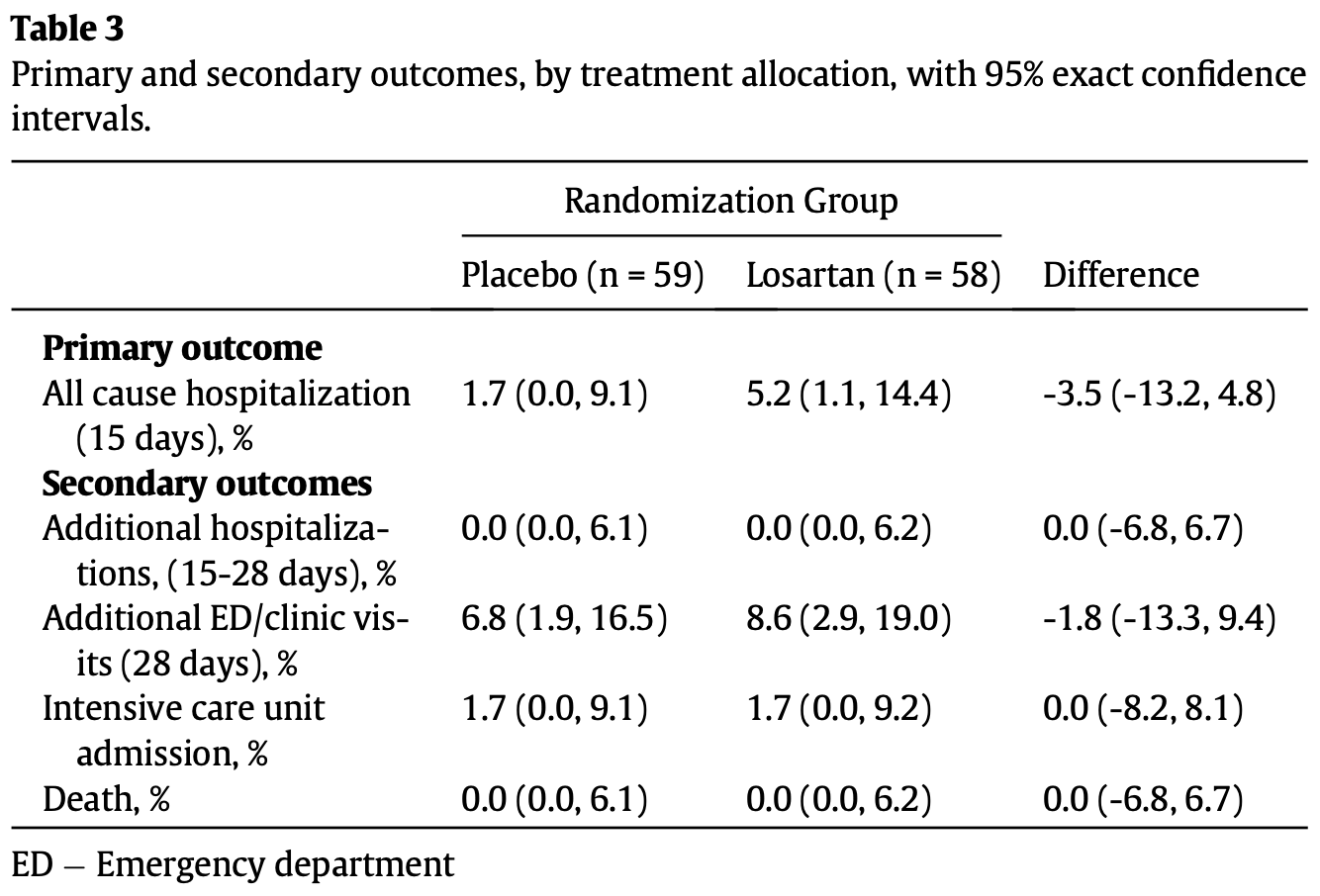
RCT 117 symptomatic outpatients showing no significant difference in hospitalization, functional status, dyspnea, or viral load with losartan treatment. The trial was terminated early due to low event rates. Losartan 25mg twice daily for 10 days.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of ICU admission, 1.7% higher, RR 1.02, p = 1.00, treatment 1 of 58 (1.7%), control 1 of 59 (1.7%).
|
|
risk of hospitalization, 205.2% higher, RR 3.05, p = 0.36, treatment 3 of 58 (5.2%), control 1 of 59 (1.7%).
|
|
additional ED/clinic visits, 27.2% higher, RR 1.27, p = 0.74, treatment 5 of 58 (8.6%), control 4 of 59 (6.8%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Puskarich et al., 31 Jul 2021, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 19 authors, study period April 2020 - November 2020, trial NCT04311177 (history).
Contact: mike-em@umn.edu.
A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19
eClinicalMedicine, doi:10.1016/j.eclinm.2021.100957
Background: The SARS-CoV-2 virus enters cells via Angiotensin-converting enzyme 2 (ACE2), disrupting the renin-angiotensin-aldosterone axis, potentially contributing to lung injury. Treatment with angiotensin receptor blockers (ARBs), such as losartan, may mitigate these effects, though induction of ACE2 could increase viral entry, replication, and worsen disease. Methods: This study represents a placebo-controlled blinded randomized clinical trial (RCT) to test the efficacy of losartan on outpatients with COVID-19 across three hospital systems with numerous community sites in Minnesota, U.S. Participants included symptomatic outpatients with COVID-19 not already taking ACE-inhibitors or ARBs, enrolled within 7 days of symptom onset. Patients were randomized to 1:1 losartan (25 mg orally twice daily unless estimated glomerular filtration rate, eGFR, was reduced, when dosing was reduced to once daily) versus placebo for 10 days, and all patients and outcome assesors were blinded. The primary outcome was all-cause hospitalization within 15 days. Secondary outcomes included functional status, dyspnea, temperature, and viral load. (clinicatrials.gov, NCT04311177, closed to new participants) Findings: From April to November 2020, 117 participants were randomized 58 to losartan and 59 to placebo, and all were analyzed under intent to treat principles. The primary outcome did not differ significantly between the two arms based on Barnard's test [losartan arm: 3 events (5.2% 95% CI 1.1, 14.4%) versus placebo arm: 1 event (1.7%; 95% CI 0.0, 9.1%)]; proportion difference -3.5% (95% CI -13.2, 4.8%); p = 0.32]. Viral loads were not statistically different between treatment groups at any time point. Adverse events per 10 patient days did not differ signifcantly [0.33 (95% CI 0.22À0.49) for losartan vs. 0.37 (95% CI 0.25À0.55) for placebo]. Due to a lower than expected hospitalization rate and low likelihood of a clinically important treatment effect, the trial was terminated early. Interpretation: In this multicenter blinded RCT for outpatients with mild symptomatic COVID-19 disease, losartan did not reduce hospitalizations, though assessment was limited by low event rate. Importantly, viral load was not statistically affected by treatment. This study does not support initiation of losartan for low-risk outpatients.
Declaration of Competing Interest The authors have no financial conflicts of interest to disclose. All the authors report grants from Minnesota Partnership for Biotechnology and Medical Genomics during the conduct of the study. MAP reports also grants from Bill and Melinda Gates Foundation and grants from NHLBI, outside the submitted work.
CRediT authorship contribution statement
References
Asch, Sheils, Islam, Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic, JAMA Intern Med, doi:10.1001/jamainternmed.2020.8193
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Biehl, Kashyap, Ahmed, Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: a population-based study, Crit Care
Cdc, Symptoms of coronavirus
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Duarte, Pelorosso, Nicolosi, Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial, medRxiv
Fang, Karakiulakis, Roth, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Lancet Respir Med, doi:10.1016/s2213-2600(20)30116-8
Fleiss, Tytun, Ury, A simple approximation for calculating sample sizes for comparing independent proportions, Biometrics
Group, Horby, Lim, Dexamethasone in hospitalized patients with Covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Harris, Low demand for antibody drugs against COVID-19, NPR
Hinchcliff, Beaumont, Thavarajah, Validity of two new patient reported outcome measures in systemic sclerosis: the PROMIS-29 profile and the FACIT-dyspnea, Arthritis Care Res
Imai, Kuba, Penninger, The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice, Exp Physiol
Imai, Kuba, Rao, Angiotensin-converting enzyme 2 protects from severe acute lung failure, Nature
Ingraham, Barakat, Reilkoff, Understanding the renin-angiotensinaldosterone-SARS-CoV axis: a comprehensive review, Eur Respir J, doi:10.1183/13993003.00912-2020
Ishiyama, Gallagher, Averill, Tallant, Brosnihan et al., Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors, Hypertension
Jaton, Stang, Biros, The use of electronic consent for COVID-19 clinical trials: lessons for emergency care research during a pandemic and beyond, Acad Emerg Med
Jenkinson, Layte, Jenkinson, A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies?, J Public Health Med, doi:10.1093/oxfordjournals.pubmed.a024606
Lindholt, Jørgensen, Bor, Petersen, Willingness to use an approved COVID-19 vaccine: cross-national evidence on levels and individual-level predictors, doi:10.31234/osf.io/8kn5f
Liu, Yang, Zhang, Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury, Sci China Life Sci, doi:10.1007/s11427-020-1643-8
Mancia, Rea, Ludergnani, Apolone, Corrao, Renin-angiotensin-aldosterone system blockers and the risk of Covid-19, N Engl J Med
Nelson, Auch, Schomaker, Analytical validation of a COVID-19 qRT-PCR detection assay using a 384-well format and three extraction methods, Cold Spring Harbor Lab, doi:10.1101/2020.04.02.022186
Reynolds, Adhikari, Pulgarin, Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19, N Engl J Med
Ruiz-Ortega, Ruperez, Esteban, Rodriguez-Vita, Sanchez-Lopez et al., Modulation of angiotensin II effects, a potential novel approach to inflammatory and immune diseases, Curr Med Chem Anti-Inflamm Anti-Allergy Agents, doi:10.2174/1568014033483626
Schulz, Altman, Moher, Group, CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials, BMJ
Sica, Gehr, Ghosh, Clinical pharmacokinetics of losartan, Clin Pharmacokinet
Team, A Language and Environment for Statistical Computing
Tignanelli, Ingraham, Sparks, Antihypertensive drugs and risk of COVID-19?, Lancet Respir Med
Wallace, James, Silver, Rapid transmission of severe acute respiratory syndrome coronavirus 2 in detention facility, Louisiana, USA, May, Emerg Infect Dis, doi:10.3201/eid2702.204158
Walter, Han, Guyatt, A systematic survey of randomised trials that stopped early for reasons of futility, BMC Med Res Methodol
Website, None
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Wu, Mcgoogan, Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention, JAMA
Wu, Ye, Mullick, Effects of renin-angiotensin inhibition on ACE2 (Angiotensin-Converting Enzyme 2) and TMPRSS2 (Transmembrane protease serine 2) expression: insights Into COVID-19, Hypertension
Yount, Atwood, Donohue, Responsiveness of PROMIS Ò to change in chronic obstructive pulmonary disease, J Patient Rep Outcomes
Zou, Yan, Shu, Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections, Nat Commun
DOI record:
{
"DOI": "10.1016/j.eclinm.2021.100957",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2021.100957",
"alternative-id": [
"S2589537021002376"
],
"article-number": "100957",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2021.100957"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6358-4670",
"affiliation": [],
"authenticated-orcid": false,
"family": "Puskarich",
"given": "Michael A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cummins",
"given": "Nathan W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ingraham",
"given": "Nicholas E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wacker",
"given": "David A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reilkoff",
"given": "Ronald A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7141-0256",
"affiliation": [],
"authenticated-orcid": false,
"family": "Driver",
"given": "Brian E",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4244-4753",
"affiliation": [],
"authenticated-orcid": false,
"family": "Biros",
"given": "Michelle H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bellolio",
"given": "Fernanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chipman",
"given": "Jeffrey G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nelson",
"given": "Andrew C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beckman",
"given": "Kenneth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Langlois",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bold",
"given": "Tyler",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6902-9149",
"affiliation": [],
"authenticated-orcid": false,
"family": "Aliota",
"given": "Matthew T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schacker",
"given": "Timothy W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Voelker",
"given": "Helen T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murray",
"given": "Thomas A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koopmeiners",
"given": "Joseph S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tignanelli",
"given": "Christopher J.",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
6,
17
]
],
"date-time": "2021-06-17T22:36:19Z",
"timestamp": 1623969379000
},
"deposited": {
"date-parts": [
[
2022,
12,
14
]
],
"date-time": "2022-12-14T08:13:29Z",
"timestamp": 1671005609000
},
"indexed": {
"date-parts": [
[
2024,
5,
13
]
],
"date-time": "2024-05-13T12:43:16Z",
"timestamp": 1715604196539
},
"is-referenced-by-count": 54,
"issued": {
"date-parts": [
[
2021,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
26
]
],
"date-time": "2021-05-26T00:00:00Z",
"timestamp": 1621987200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021002376?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021002376?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100957",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.eclinm.2021.100957_bib0001",
"unstructured": "Coronavirus update (Live): 86,321,500 cases and 1,865,798 deaths from COVID-19 virus pandemic - Worldometer. Accessed January 5, 2021. https://www.worldometers.info/coronavirus/."
},
{
"DOI": "10.1183/13993003.00912-2020",
"article-title": "Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review",
"author": "Ingraham",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "10.1016/j.eclinm.2021.100957_bib0002",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1007/s11427-020-1643-8",
"article-title": "Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "364",
"issue": "3",
"journal-title": "Sci China Life Sci",
"key": "10.1016/j.eclinm.2021.100957_bib0003",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1038/nature03712",
"article-title": "Angiotensin-converting enzyme 2 protects from severe acute lung failure",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "112",
"issue": "7047",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2021.100957_bib0004",
"volume": "436",
"year": "2005"
},
{
"DOI": "10.1113/expphysiol.2007.040048",
"article-title": "The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "543",
"issue": "5",
"journal-title": "Exp Physiol",
"key": "10.1016/j.eclinm.2021.100957_bib0005",
"volume": "93",
"year": "2008"
},
{
"DOI": "10.1038/ncomms4594",
"article-title": "Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "3594",
"journal-title": "Nat Commun",
"key": "10.1016/j.eclinm.2021.100957_bib0006",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1136/bmj.c332",
"article-title": "CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials",
"author": "Schulz",
"doi-asserted-by": "crossref",
"first-page": "c332",
"journal-title": "BMJ",
"key": "10.1016/j.eclinm.2021.100957_bib0007",
"volume": "340",
"year": "2010"
},
{
"key": "10.1016/j.eclinm.2021.100957_bib0008",
"unstructured": "CDC. Symptoms of coronavirus. Published January 4, 2021. Accessed January 5, 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html."
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support",
"doi-asserted-by": "crossref",
"first-page": "377",
"issue": "2",
"journal-title": "J Biomed Inform",
"key": "10.1016/j.eclinm.2021.100957_bib0009",
"volume": "42",
"year": "2009"
},
{
"article-title": "The REDCap consortium: building an international community of software platform partners",
"journal-title": "J Biomed Inform",
"key": "10.1016/j.eclinm.2021.100957_bib0010",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.2165/00003088-200544080-00003",
"article-title": "Clinical pharmacokinetics of losartan",
"author": "Sica",
"doi-asserted-by": "crossref",
"first-page": "797",
"issue": "8",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/j.eclinm.2021.100957_bib0011",
"volume": "44",
"year": "2005"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 - final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100957_bib0012",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1186/s13054-015-1062-y",
"article-title": "Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: a population-based study",
"author": "Biehl",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Crit Care",
"key": "10.1016/j.eclinm.2021.100957_bib0013",
"volume": "19",
"year": "2015"
},
{
"key": "10.1016/j.eclinm.2021.100957_bib0014",
"unstructured": "Website. Accessed January 5, 2021. https://www.jstor.org/stable/3766749?seq=1"
},
{
"DOI": "10.1002/acr.20591",
"article-title": "Validity of two new patient reported outcome measures in systemic sclerosis: the PROMIS-29 profile and the FACIT-dyspnea",
"author": "Hinchcliff",
"doi-asserted-by": "crossref",
"first-page": "1620",
"issue": "11",
"journal-title": "Arthritis Care Res",
"key": "10.1016/j.eclinm.2021.100957_bib0015",
"volume": "63",
"year": "2011"
},
{
"DOI": "10.1186/s41687-019-0155-9",
"article-title": "Responsiveness of PROMIS® to change in chronic obstructive pulmonary disease",
"author": "Yount",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "J Patient Rep Outcomes",
"key": "10.1016/j.eclinm.2021.100957_bib0016",
"volume": "3",
"year": "2019"
},
{
"DOI": "10.2174/1568014033483626",
"article-title": "Modulation of angiotensin II effects, a potential novel approach to inflammatory and immune diseases",
"author": "Ruiz-Ortega",
"doi-asserted-by": "crossref",
"first-page": "379",
"issue": "4",
"journal-title": "Curr Med Chem Anti-Inflamm Anti-Allergy Agents",
"key": "10.1016/j.eclinm.2021.100957_bib0017",
"volume": "2",
"year": "2003"
},
{
"article-title": "Analytical validation of a COVID-19 qRT-PCR detection assay using a 384-well format and three extraction methods",
"author": "Nelson",
"journal-title": "Cold Spring Harbor Lab",
"key": "10.1016/j.eclinm.2021.100957_bib0018",
"year": "2020"
},
{
"author": "Core Team",
"key": "10.1016/j.eclinm.2021.100957_bib0019",
"series-title": "R: A Language and Environment for Statistical Computing",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.100957_bib0020",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.2307/2529990",
"article-title": "A simple approximation for calculating sample sizes for comparing independent proportions",
"author": "Fleiss",
"doi-asserted-by": "crossref",
"first-page": "343",
"journal-title": "Biometrics",
"key": "10.1016/j.eclinm.2021.100957_bib0021",
"volume": "36",
"year": "1980"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19 - preliminary report",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100957_bib0022",
"year": "2020"
},
{
"article-title": "Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic",
"author": "Asch",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.eclinm.2021.100957_bib0023",
"volume": "22",
"year": "2020"
},
{
"article-title": "An EUA for bamlanivimab—a monoclonal antibody for COVID-19",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.100957_bib0024",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100957_bib0025",
"year": "2020"
},
{
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100957_bib0026",
"volume": "17",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2021.100957_bib0027",
"unstructured": "Harris R. Low demand for antibody drugs against COVID-19. NPR. Published December 22, 2020. Accessed January 5, 2021. https://www.npr.org/sections/health-shots/2020/12/22/948874701/low-demand-for-antibody-drugs-against-covid-19."
},
{
"DOI": "10.31234/osf.io/8kn5f",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100957_bib0028",
"unstructured": "Lindholt M.F., Jørgensen F.J., Bor A., Petersen MB. Willingness to use an approved COVID-19 vaccine: cross-national evidence on levels and individual-level predictors. doi:10.31234/osf.io/8kn5f"
},
{
"DOI": "10.3201/eid2702.204158",
"article-title": "Rapid transmission of severe acute respiratory syndrome coronavirus 2 in detention facility, Louisiana, USA, May-June, 2020",
"author": "Wallace",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Emerg Infect Dis",
"key": "10.1016/j.eclinm.2021.100957_bib0029",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1093/oxfordjournals.pubmed.a024606",
"article-title": "A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies?",
"author": "Jenkinson",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "J Public Health Med",
"key": "10.1016/j.eclinm.2021.100957_bib0030",
"volume": "19",
"year": "1997"
},
{
"key": "10.1016/j.eclinm.2021.100957_bib0031",
"unstructured": "[No title]. Accessed January 6, 2021. http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Dyspnea_Scoring_Manual.pdf."
},
{
"DOI": "10.1161/01.HYP.0000124667.34652.1a",
"article-title": "Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors",
"author": "Ishiyama",
"doi-asserted-by": "crossref",
"first-page": "970",
"issue": "5",
"journal-title": "Hypertension",
"key": "10.1016/j.eclinm.2021.100957_bib0032",
"volume": "43",
"year": "2004"
},
{
"DOI": "10.1016/S2213-2600(20)30153-3",
"article-title": "Antihypertensive drugs and risk of COVID-19?",
"author": "Tignanelli",
"doi-asserted-by": "crossref",
"first-page": "e30",
"issue": "5",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.eclinm.2021.100957_bib0033",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30116-8",
"article-title": "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?",
"author": "Fang",
"doi-asserted-by": "crossref",
"first-page": "e21",
"issue": "4",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.eclinm.2021.100957_bib0034",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.120.15782",
"article-title": "Effects of renin-angiotensin inhibition on ACE2 (Angiotensin-Converting Enzyme 2) and TMPRSS2 (Transmembrane protease serine 2) expression: insights Into COVID-19",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "e29",
"issue": "4",
"journal-title": "Hypertension",
"key": "10.1016/j.eclinm.2021.100957_bib0035",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1101/2020.08.04.20167205",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100957_bib0036",
"unstructured": "Duarte M., Pelorosso F.G., Nicolosi L., et al. Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial. Preliminary report. medRxiv. Published online August 13, 2020:2020.08.04.20167205."
},
{
"key": "10.1016/j.eclinm.2021.100957_bib0037",
"unstructured": "First randomized trial backs safety of common heart drugs in COVID-19 patients. Accessed January 5, 2021. https://www.escardio.org/The-ESC/Press-Office/Press-releases/LOPES."
},
{
"DOI": "10.1056/NEJMoa2006923",
"article-title": "Renin-angiotensin-aldosterone system blockers and the risk of Covid-19",
"author": "Mancia",
"doi-asserted-by": "crossref",
"first-page": "2431",
"issue": "25",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100957_bib0038",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2008975",
"article-title": "Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19",
"author": "Reynolds",
"doi-asserted-by": "crossref",
"first-page": "2441",
"issue": "25",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100957_bib0039",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1111/acem.14141",
"article-title": "The use of electronic consent for COVID-19 clinical trials: lessons for emergency care research during a pandemic and beyond",
"author": "Jaton",
"doi-asserted-by": "crossref",
"first-page": "1183",
"issue": "11",
"journal-title": "Acad Emerg Med",
"key": "10.1016/j.eclinm.2021.100957_bib0040",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1186/s12874-020-0899-1",
"article-title": "A systematic survey of randomised trials that stopped early for reasons of futility",
"author": "Walter",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "BMC Med Res Methodol",
"key": "10.1016/j.eclinm.2021.100957_bib0041",
"volume": "20",
"year": "2020"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537021002376"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "37"
}