Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID™ for Post-Exposure Prophylactic Use
, Press Release, EPIC-PEP, NCT05047601, Apr 2022
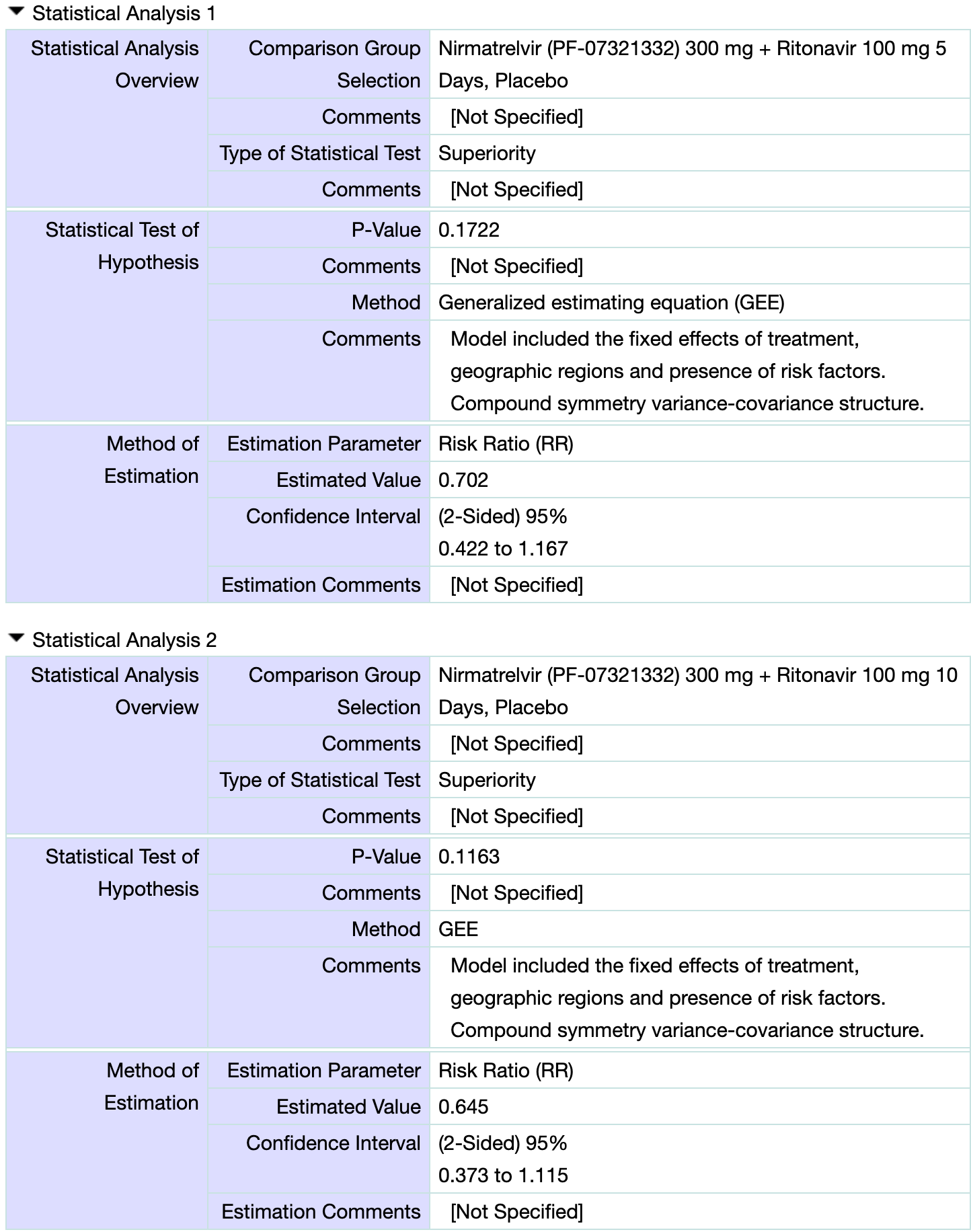
PEP RCT showing no significant difference with paxlovid post-exposure prophylaxis. Results from1.
|
risk of hospitalization, 66.5% lower, RR 0.33, p = 1.00, treatment 0 of 830 (0.0%), control 1 of 840 (0.1%), NNT 840, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 10 day treatment.
|
|
risk of hospitalization, 66.7% lower, RR 0.33, p = 0.50, treatment 0 of 844 (0.0%), control 1 of 840 (0.1%), NNT 840, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 5 day treatment.
|
|
risk of severe case, 25.2% lower, RR 0.75, p = 0.42, treatment 17 of 830 (2.0%), control 23 of 840 (2.7%), NNT 145, 10 day treatment.
|
|
risk of severe case, 22.1% lower, RR 0.78, p = 0.43, treatment 18 of 844 (2.1%), control 23 of 840 (2.7%), NNT 165, 5 day treatment.
|
|
medical visit, 15.3% lower, RR 0.85, p = 0.20, treatment 830, control 840, 10 day treatment.
|
|
medical visit, 3.1% lower, RR 0.97, p = 0.81, treatment 844, control 840, 5 day treatment.
|
|
risk of symptomatic case, 35.5% lower, RR 0.65, p = 0.12, treatment 830, control 840, 10 day treatment, PCR- at baseline.
|
|
risk of symptomatic case, 29.8% lower, RR 0.70, p = 0.17, treatment 844, control 840, 5 day treatment, PCR- at baseline.
|
|
risk of symptomatic case, 4.7% lower, RR 0.95, p = 0.82, treatment 887, control 873, 10 day treatment.
|
|
risk of symptomatic case, 27.4% lower, RR 0.73, p = 0.13, treatment 889, control 873, 5 day treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pfizer et al., 29 Apr 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, preprint, 1 author, study period 9 September, 2021 - 12 April, 2022, trial NCT05047601 (history) (EPIC-PEP).