The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants
et al., medRxiv, doi:10.1101/2022.11.14.22282195, Nov 2022
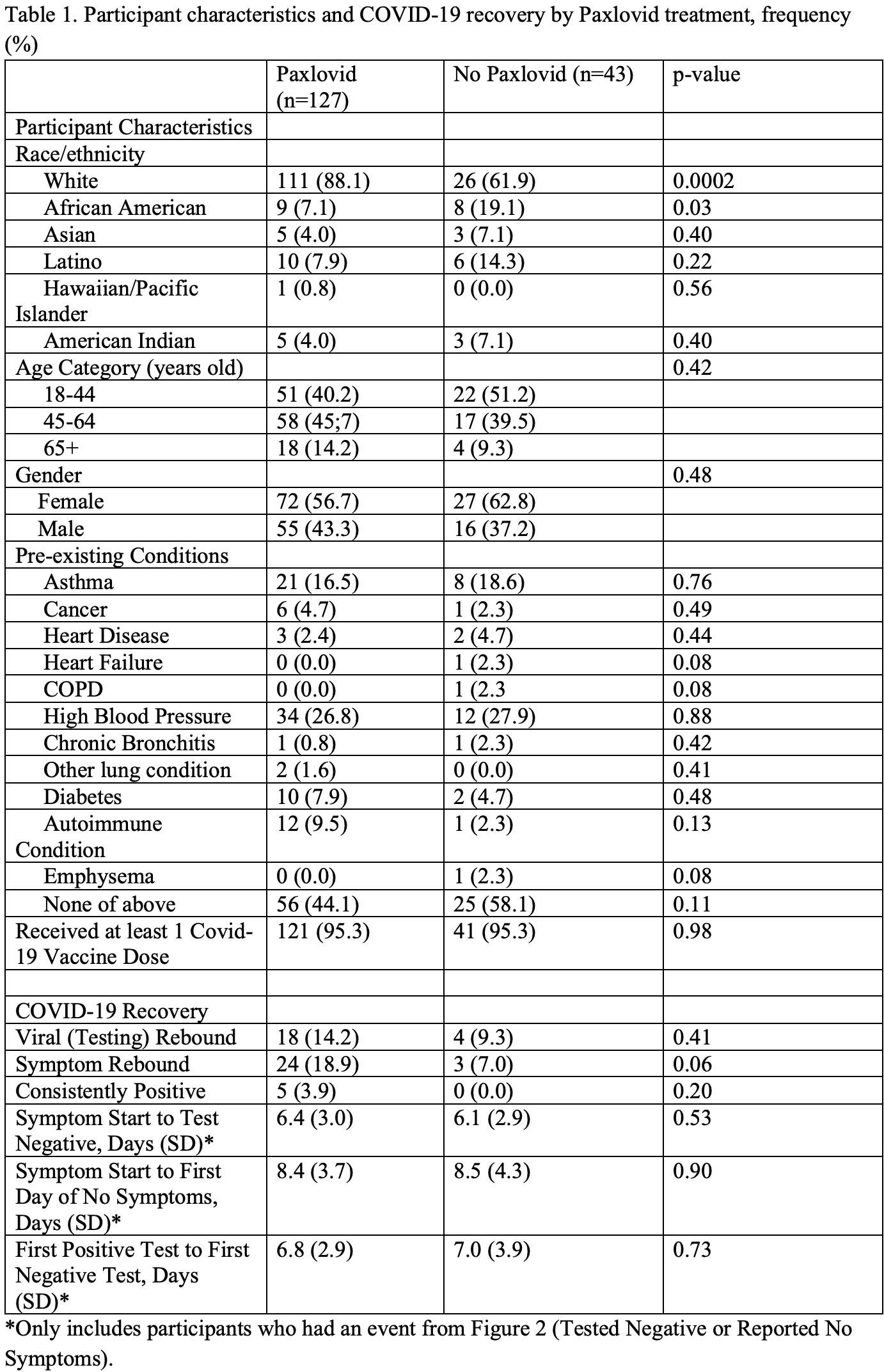
Prospective study of 170 COVID-19 patients in the USA, showing no significant difference in symptomatic and viral recovery times, and higher risk of symptomatic rebound, without statistical significance. There were more elderly patients in the paxlovid group.
5 paxlovid patients did not test negative during the 16 day daily followup, compared to zero for control. Only patients testing negative or reporting no symptoms were included in the recovery time analyses. KM curves show no significant difference in viral or symptomatic recovery.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of no recovery, 1.2% lower, RR 0.99, p = 0.90.
|
|
risk of no viral clearance, 2.9% lower, RR 0.97, p = 0.73.
|
|
symptomatic rebound, 170.9% higher, RR 2.71, p = 0.06, treatment 24 of 127 (18.9%), control 3 of 43 (7.0%).
|
|
viral rebound, 52.4% higher, RR 1.52, p = 0.41, treatment 18 of 127 (14.2%), control 4 of 43 (9.3%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pandit et al., 15 Nov 2022, prospective, USA, preprint, 8 authors, study period 4 August, 2022 - 1 November, 2022.
Contact: jpandit@scripps.edu.
The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants
doi:10.1101/2022.11.14.22282195
Introduction: The uptake of Paxlovid in individuals infected with COVID-19 has been significantly limited by concerns around the Paxlovid rebound phenomenon despite the scarcity of evidence around its epidemiology. The purpose of this study was to prospectively compare the epidemiology of Paxlovid rebound in treated and untreated participants with acute COVID-19 infection Methods: We designed a decentralized, digital, prospective observational study in which participants who tested positive for COVID-19 using eMed Test-to-Treat telehealth kits and were clinically eligible for Paxlovid were recruited to be evaluated for viral and symptom clearance, as well as rebound. Participants were assigned to a Paxlovid or control group based on their decision to take Paxlovid. Following initial diagnosis based on a telehealth proctored test both groups were provided 12 telehealth proctored rapid antigen home tests and asked to test on a regular frequent schedule for 16 days and answer symptom surveys. Viral rebound based on test results and COVID-19 symptom rebound based on patient reported symptoms were evaluated. Results: Viral rebound incidence was 14.2% in the Paxlovid group (n=127) and 9.3% in the control group (n=43). COVID-19 symptom rebound incidence was higher in the Paxlovid group (18.9%) compared to the control group (7.0%). There were no notable differences in viral rebound by age, gender, pre-existing conditions, or major symptom groups during the acute phase or at the 1-month interval. Conclusion: This preliminary report of our prospective study suggests that rebound after clearance of test positivity or symptom resolution is higher than previously reported. However, we observed a similar rate of rebound in both in the Paxlovid and control groups. Large studies with diverse participants and extended follow-up are needed to better understand the rebound phenomena.
References
Alshanqeeti, Bhargava, COVID-19 Rebound After Paxlovid Treatment: A Case Series and Review of Literature, Cureus
Anderson, Caubel, Rusnak, Investigators, Nirmatrelvir-Ritonavir and Viral Load Rebound in Covid-19, N Engl J Med
Callaway, COVID rebound is surprisingly common -even without Paxlovid, Nature
Carlin, Clark, Chaillon, Garretson, Bray et al., Virologic and Immunologic Characterization of COVID-19 Recrudescence after Nirmatrelvir/Ritonavir Treatment, Res Sq
Cdc, COVID-19 Rebound After Paxlovid Treatment: The Centers for Disease Control and Prevention
Charness, Gupta, Stack, Strymish, Lindy, Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment, N Engl J Med
Coulson, Adams, Gray, Evans, COVID-19 "Rebound" associated with nirmatrelvir/ritonavir pre-hospital therapy, J Infect
Deo, Choudhary, Moser, Ritz, Daar et al., Viral and Symptom Rebound in Untreated COVID-19 Infection
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis
Farley, FDA Updates on Paxlovid for Health Care Providers, U.S. Food & Drug Administration
Ganatra, Dani, Ahmad, Kumar, Shah et al., Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19, Clin Infect Dis
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Harris, Taylor, Minor, Elliott, Fernandez et al., The REDCap consortium: Building an international community of software platform partners, J Biomed Inform
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Peluso, Anglin, Durstenfeld, Martin, Kelly et al., Effect of Oral Nirmatrelvir on Long COVID Symptoms: 4 Cases and Rationale for Systematic Studies, Pathog Immun
Pfizer, Pfizer To Provide, Government with an Additional 10 Million Treatment Courses of its Oral Therapy to Help Combat COVID-19
Rubin, From Positive to Negative to Positive Again-The Mystery of Why COVID-19
Smith, Li, Moser, Yeh, Currier et al., Recurrence of Symptoms Following a 2-Day Symptom Free Period in Patients With COVID-19, JAMA Netw Open
Wang, Berger, Davis, Kaelber, Volkow et al., COVID-19 rebound after Paxlovid and Molnupiravir during January
Wang, Chen, Zhao, Feng, Rapid COVID-19 rebound in a severe COVID-19 patient during 20-day course of Paxlovid, J Infect
Wang, Volkow, Davis, Berger, Kaelber et al., COVID-19 rebound after Paxlovid treatment during, Omicron BA
Xie, Choi, Al-Aly, Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19, medRxiv
DOI record:
{
"DOI": "10.1101/2022.11.14.22282195",
"URL": "http://dx.doi.org/10.1101/2022.11.14.22282195",
"abstract": "<jats:p>Introduction: The uptake of Paxlovid in individuals infected with COVID-19 has been significantly limited by concerns around the Paxlovid rebound phenomenon despite the scarcity of evidence around its epidemiology. The purpose of this study was to prospectively compare the epidemiology of Paxlovid rebound in treated and untreated participants with acute COVID-19 infection Methods: We designed a digital, prospective observational study, which included participants who tested positive for COVID-19 and were clinically eligible for Paxlovid. Participants were assigned to a Paxlovid or control group based on their decision to take the medication. Both groups were provided 12 rapid antigen tests and asked to test and answer symptom surveys on a regular frequent schedule for 16 days. Viral rebound based on test results and COVID-19 symptom rebound based on patient reported symptoms were evaluated. Results: Viral rebound incidence was 14.2% in the Paxlovid group (n=127) and 9.3% in the control group (n=43). COVID-19 symptom rebound incidence was higher in the Paxlovid group (18.9%) compared to the control group (7.0%). There were no notable differences in viral rebound by age, gender, pre-existing conditions, or major symptom groups during the acute phase or at the 1-month interval. Conclusion: This preliminary report of our prospective study suggests that rebound after clearance of test positivity or symptom resolution is higher than previously reported. However, we observed a similar rate of rebound in both in the Paxlovid and control groups. Large studies with diverse participants and extended follow-up are needed to better understand the rebound phenomena.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
11,
15
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0119-0881",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pandit",
"given": "Jay A",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-3843-0842",
"affiliation": [],
"authenticated-orcid": false,
"family": "Radin",
"given": "Jennifer M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0919-9303",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chiang",
"given": "Danielle",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2650-4357",
"affiliation": [],
"authenticated-orcid": false,
"family": "Spencer",
"given": "Emily G",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1419-6131",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pawelek",
"given": "Jeff B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diwan",
"given": "Mira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roumani",
"given": "Leila",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0674-5762",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mina",
"given": "Michael J",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
15
]
],
"date-time": "2022-11-15T18:49:34Z",
"timestamp": 1668538174000
},
"deposited": {
"date-parts": [
[
2022,
11,
15
]
],
"date-time": "2022-11-15T18:49:34Z",
"timestamp": 1668538174000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
11,
16
]
],
"date-time": "2022-11-16T05:52:36Z",
"timestamp": 1668577956421
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
15
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.11.14.22282195",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
11,
15
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
11,
15
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.11.14.22282195"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants",
"type": "posted-content"
}