Oral mouthwashes for asymptomatic to mildly symptomatic adults with COVID-19 and salivary viral load: a randomized, placebo-controlled, open-label clinical trial
et al., BMC Oral Health, doi:10.1186/s12903-024-04246-1, jRCTs051220107, Apr 2024
58th treatment shown to reduce risk in
September 2025, now with p = 0.0035 from 4 studies.
Lower risk for recovery and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
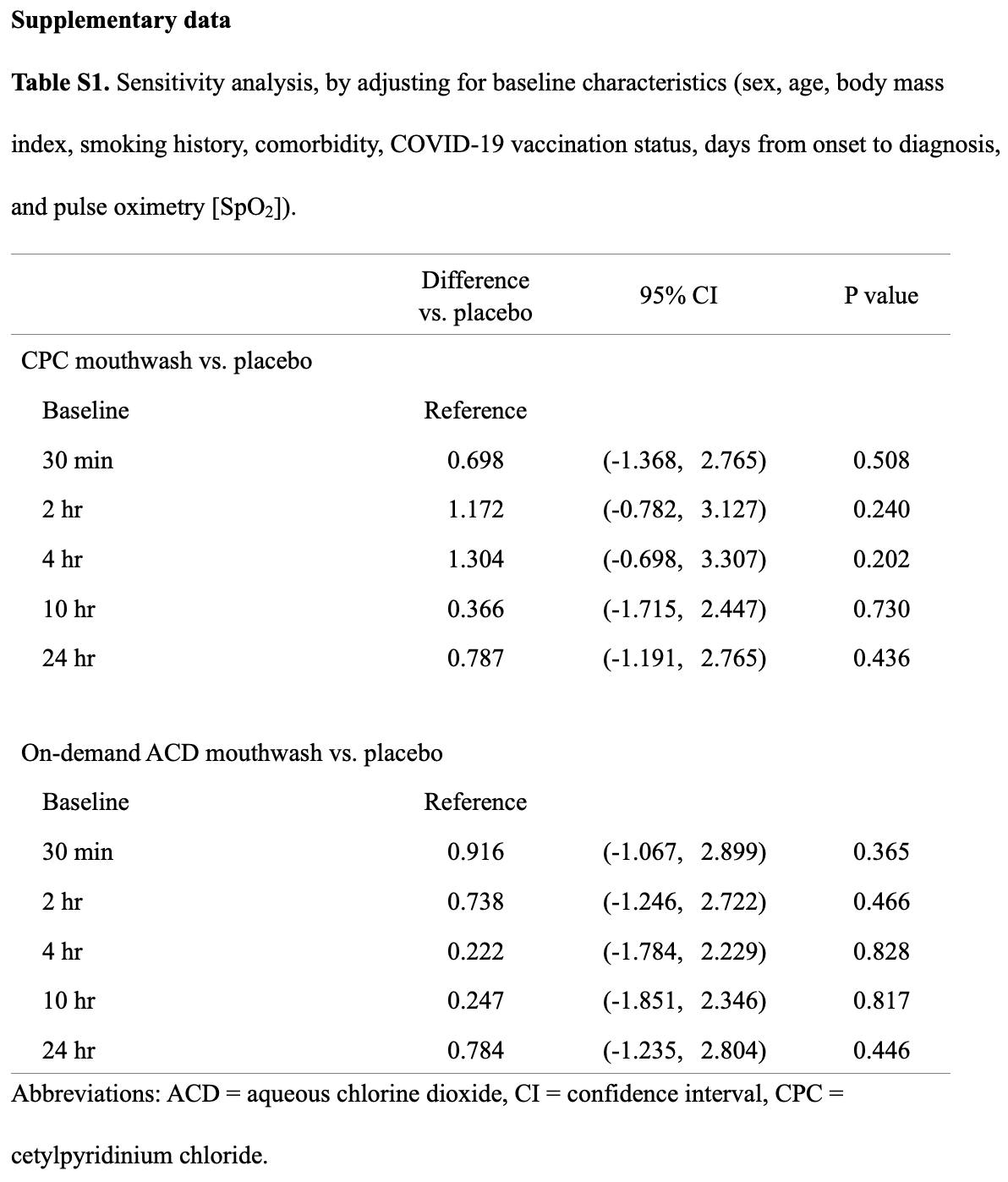
RCT 90 low-risk asymptomatic to mildly symptomatic COVID-19 patients showing no significant difference in salivary viral load with cetylpyridinium chloride or on-demand aqueous chlorine dioxide mouthwash. Both treatments increased Ct values at all time intervals more than placebo, but without statistical significance.
The baseline Ct values are very high, especially in the placebo (38.0) and CPC (38.6) groups, indicating low viral loads and limited room for improvement in short-term Ct values. Treatment may minimize progression, however authors only follow patients for one day. Authors report only relative changes, therefore it is unknown if viral load increased or decreased in each arm.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
Onozuka et al., 25 Apr 2024, Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, 10 authors, study period 7 November, 2022 - 19 January, 2023, trial jRCTs051220107.
Contact: onozukad@hp-infect.med.osaka-u.ac.jp, kutsuna@hp-infect.med.osaka-u.ac.jp.
Oral mouthwashes for asymptomatic to mildly symptomatic adults with COVID-19 and salivary viral load: a randomized, placebo-controlled, open-label clinical trial
BMC Oral Health, doi:10.1186/s12903-024-04246-1
Background Recent randomized clinical trials suggest that the effect of using cetylpyridinium chloride (CPC) mouthwashes on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in COVID-19 patients has been inconsistent. Additionally, no clinical study has investigated the effectiveness of on-demand aqueous chlorine dioxide mouthwash against COVID-19.
Methods We performed a randomized, placebo-controlled, open-label clinical trial to assess for any effects of using mouthwash on the salivary SARS-CoV-2 viral load among asymptomatic to mildly symptomatic adult COVID-19-positive patients. Patients were randomized to receive either 20 mL of 0.05% CPC, 10 mL of 0.01% on-demand aqueous chlorine dioxide, or 20 mL of placebo mouthwash (purified water) in a 1:1:1 ratio. The primary endpoint was the cycle threshold (Ct) values employed for SARS-CoV-2 salivary viral load estimation. We used linear mixed-effects models to assess for any effect of the mouthwashes on SARS-CoV-2 salivary viral load.
Results Of a total of 96 eligible participants enrolled from November 7, 2022, to January 19, 2023, 90 were accepted for the primary analysis. The use of 0.05% CPC mouthwash was not shown to be superior to placebo in change from baseline salivary Ct value at 30 min (difference vs. placebo, 0.640; 95% confidence interval [CI], -1.425 to 2.706; P = 0.543); 2 h (difference vs. placebo, 1.158; 95% CI, -0.797 to 3.112; P = 0.246); 4 h (difference vs. placebo, 1.283; 95% CI, -0.719 to 3.285; P = 0.209); 10 h (difference vs. placebo, 0.304; 95% CI, -1.777 to 2.385; P = 0.775); or 24 h (difference vs. placebo, 0.782; 95% CI, -1.195 to 2.759; P = 0.438). The use of 0.01% on-demand aqueous chlorine dioxide mouthwash was also not shown to be superior to placebo in change from baseline salivary Ct value at 30 min (difference vs. placebo, 0.905; 95% CI, -1.079 to 2.888; P = 0.371); 2 h (difference vs. placebo, 0.709; 95% CI, -1.275 to 2.693; P = 0.483); 4 h (difference vs. placebo, 0.220; 95% CI, -1.787 to 2.226; P = 0.830); 10 h (difference vs. placebo, 0.198; 95% CI, -1.901 to 2.296; P = 0.854); or 24 h (difference vs. placebo, 0.784; 95% CI, -1.236 to 2.804; P = 0.447).
Supplementary Information The online version contains supplementary material available at https://doi. org/10.1186/s12903-024-04246-1.
Supplementary Material 1 Author contributions S.K supervised the project. D.O., S.T., and S.K. made substantial contributions to conception and design. D.O., S.T., H.M., H.Y., S.H., S.Y., R.M.S., and K.K. collected data. K.S. conducted laboratory test. D.O. analyzed the data, wrote the first draft of the manuscript, and interpreted the results. D.O., S.T., H.M., H.Y., S.H., S.Y., R.M.S., K.S., K.K., and S.K. contributed to critical revision of the manuscript. All authors were involved in data interpretation and made meaningful contributions to the final submitted manuscript.
Data availability The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations Ethics approval and consent to participate This open-label, randomized, placebo-controlled clinical trial was approved by the Ethical Committee of Osaka University (No. S22003) on 6/10/2022, and was registered with the Japan Registry of Clinical Trials (jRCT) (No. jRCTs051220107) on 18/10/2022. This trial was conducted in compliance with the provisions of the Declaration of Helsinki, Good Clinical Practice Guidelines, and local regulatory requirements. Patient registration was conducted from 7/11/2022 to 19/1/2023 in Osaka, Japan, and all of the participants provided written informed consent.
Consent for..
References
Alemany, Perez-Zsolt, Raich-Regue, Munoz-Basagoiti, Ouchi et al., Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: a double-blind Randomized Clinical Trial, J Dent Res
Arevalo-Rodriguez, Buitrago-Garcia, Simancas-Racines, Zambrano-Achig, Campo et al., False-negative results of initial RT-PCR assays for COVID-19: a systematic review, PLoS ONE
Bano-Polo, Martinez-Gil, Del Pino, Massoli, Mingarro et al., Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles, J Oral Microbiol
Bonn, Rohrhofer, Audebert, Lang, Auer et al., Efficacy of a Mouthwash Containing CHX and CPC in SARS-CoV-2-Positive patients: a Randomized Controlled Clinical Trial, J Dent Res
Brian, Weintraub, Oral health and COVID-19: increasing the need for Prevention and Access, Prev Chronic Dis
Cardenas, Campos-Bijit, Francesco, Schwarz, Cafferata et al., Electrolyzed water for the microbiologic control in the pandemic dental setting: a systematic review, BMC Oral Health
Chaudhary, Melkonyan, Meethil, Saraswat, Hall et al., Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: a randomized controlled trial, J Am Dent Assoc
Chen, Chang, The effectiveness of mouthwash against SARS-CoV-2 infection: a review of scientific and clinical evidence, J Formos Med Assoc
Chow, Shao, Wang, Lokhnygina, Sample size calculations in clinical research
Cordes, Rehrauer, Accola, Wolk, Hilfrich et al., Fully automated detection and differentiation of pandemic and endemic coronaviruses (NL63, 229E, HKU1, OC43 and SARS-CoV-2) on the hologic panther fusion, J Med Virol
Dhar, Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic, Anal Bioanal Chem
Ebrahimi, Shamshiri, Alebouyeh, Mohebbi, Effectiveness of mouthwashes on reducing SARS-CoV-2 viral load in oral cavity: a systematic review and meta-analysis, BMC Oral Health
Eggers, Koburger-Janssen, Eickmann, Zorn, In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash against Respiratory and oral tract pathogens, Infect Dis Ther
Fantozzi, Pampena, Pierangeli, Oliveto, Sorrentino et al., Efficacy of antiseptic mouthrinses against SARS-CoV-2: a prospective randomized placebo-controlled pilot study, Am J Otolaryngol
Ferrer, Barrueco, Martinez-Beneyto, Moreno, Ausina-Marquez et al., Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2, Sci Rep
Garcia-Sanchez, Pena-Cardelles, Ruiz, Robles, Ordonez-Fernandez et al., Efficacy of Pre-procedural mouthwashes against SARS-CoV-2: a systematic review of Randomized controlled trials, J Clin Med
Giulia, Viktoria, Robert, Michael, Nadine et al., Eligibility and efficacy of a CPC-and CHX-based antiviral mouthwash for the elimination of SARS-CoV-2 from the saliva: a randomized, double-blind, controlled clinical trial, J Clin Periodontol
Goldfarb, Tilley, Al-Rawahi, Srigley, Ford et al., Self-collected saline Gargle Samples as an alternative to Health Care workercollected nasopharyngeal swabs for COVID-19 diagnosis in outpatients, J Clin Microbiol
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of Coronavirus Disease 2019 in China, N Engl J Med
Hernandez-Vasquez, Barrenechea-Pulache, Comande, Azanedo, Mouthrinses and SARS-CoV-2 viral load in saliva: a living systematic review, Evid Based Dent
Hiroi, Kubota-Koketsu, Sasaki, Morikawa, Motomura et al., Infectivity assay for detection of SARS-CoV-2 in samples from patients with COVID-19, J Med Virol
Holmberg, Andersen, Adjustment for baseline characteristics in Randomized clinical trials, JAMA
Hologic, Instructions for Using the Aptima® Multitest Swab Specimen Collection Kit for Patient-Collected Specimens, Hologic
Hologic, SARS-CoV-2 Assay (Panther Fusion® System) Hologic
Huang, Perez, Kato, Mikami, Okuda et al., SARS-CoV-2 infection of the oral cavity and saliva, Nat Med
Joynt, Wu, Understanding. COVID-19: what does viral RNA load really mean?, Lancet Infect Dis
Julious, Sample sizes for clinical trials with normal data, Stat Med
Machin, Campbell, Tan, Tan, Sample sizes for clinical, laboratory and epidemiology studies
Marui, Souto, Rovai, Romito, Chambrone et al., Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review, J Am Dent Assoc
Meister, Gottsauner, Schmidt, Heinen, Todt et al., Mouthrinses against SARS-CoV-2 -high antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial, Virus Res
Mendoza, Ubillus, Bolivar, Palacios, Lopez et al., Antiviral effect of mouthwashes against SARS-COV-2: a systematic review, Saudi Dent J
Migueres, Mengelle, Dimeglio, Didier, Alvarez et al., Saliva sampling for diagnosing SARS-CoV-2 infections in symptomatic patients and asymptomatic carriers, J Clin Virol
Migueres, Vellas, Abravanel, Silva, Dimeglio et al., Testing individual and pooled saliva samples for sars-cov-2 nucleic acid: a prospective study, Diagn Microbiol Infect Dis
Munoz-Basagoiti, Perez-Zsolt, Leon, Blanc, Raich-Regue et al., Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in Vitro, J Dent Res
Noguchi, Tachibana, Inoue, Sakai, Tsujikawa et al., Safety evaluation of MA-T after ingestion in mice, Toxicology
Reis, Amaral, Mendoza, Das Gracas, Mendes-Correa et al., Can preprocedural mouthrinses reduce SARS-CoV-2 load in dental aerosols?, Med Hypotheses
Saito, Adachi, Yamayoshi, Koga, Iwatsuki-Horimoto et al., Gargle Lavage as a safe and sensitive alternative to Swab samples to Diagnose COVID-19: a Case Report in Japan, Clin Infect Dis
Sanchez Barrueco, Moreno, Martinez-Beneyto, Vazquez, Gonzalez et al., Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial, Emerg Microbes Infect
Seneviratne, Balan, Ko, Udawatte, Lai et al., Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Shibata, Konishi, The respiratory chain of Bacteria is a target of the disinfectant MA-T, BPB Rep
Shibata, Urakawa, Ono, Akeda, Sakai et al., Verification of MA-T Safety and Efficacy Against pathogens Including SARS-CoV-2, BPB Rep
Takara Bio, Instructions for Using the Aptima® Multitest Swab Specimen Collection Kit for Patient-Collected Specimens
Takeda, Sawa, Sasaki, Orba, Maishi et al., Antiviral effect of cetylpyridinium chloride in mouthwash on SARS-CoV-2, Sci Rep
Ting, Dahlkemper, Schwartz, Woodfork, Suzuki, Preprocedural viral load effects of oral antiseptics on SARS-CoV-2 in patients with COVID-19: a systematic review, Biomedicines
Urakawa, Shibata, Sogou, Takamori, Inoue et al., The Bactericidal Effect of MA-T for Factitiously contaminated and used masks, Biol Pharm Bull
Wang, Xu, Gao, Lu, Han et al., Detection of SARS-CoV-2 in different types of clinical specimens, JAMA
Welch, The significance of the difference between two means when the population variances are unequal, Biometrika
Wong, Wong, Ho, Leung, Lai, Performance evaluation of panther Fusion SARS-CoV-2 assay for detection of SARS-CoV-2 from deep throat saliva, nasopharyngeal, and lower-respiratory-tract specimens, J Med Virol
Wyllie, Fournier, Casanovas-Massana, Campbell, Tokuyama et al., Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, N Engl J Med
Xu, Li, Gan, Du, Yao, Salivary glands: potential reservoirs for COVID-19 asymptomatic infection, J Dent Res
Yazawa, Yamazaki, Saga, Itamochi, Inasaki et al., Evaluation of SARS-CoV-2 isolation in cell culture from nasal/nasopharyngeal swabs or saliva specimens of patients with COVID-19, Sci Rep
Zar, Biostatistical Analysis
Zhang, Meng, Duo, Yang, Dong et al., Efficacy of mouthwash on reducing salivary SARS-CoV-2 viral load and clinical symptoms: a systematic review and meta-analysis, BMC Infect Dis
DOI record:
{
"DOI": "10.1186/s12903-024-04246-1",
"ISSN": [
"1472-6831"
],
"URL": "http://dx.doi.org/10.1186/s12903-024-04246-1",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Recent randomized clinical trials suggest that the effect of using cetylpyridinium chloride (CPC) mouthwashes on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in COVID-19 patients has been inconsistent. Additionally, no clinical study has investigated the effectiveness of on-demand aqueous chlorine dioxide mouthwash against COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a randomized, placebo-controlled, open-label clinical trial to assess for any effects of using mouthwash on the salivary SARS-CoV-2 viral load among asymptomatic to mildly symptomatic adult COVID-19-positive patients. Patients were randomized to receive either 20 mL of 0.05% CPC, 10 mL of 0.01% on-demand aqueous chlorine dioxide, or 20 mL of placebo mouthwash (purified water) in a 1:1:1 ratio. The primary endpoint was the cycle threshold (Ct) values employed for SARS-CoV-2 salivary viral load estimation. We used linear mixed-effects models to assess for any effect of the mouthwashes on SARS-CoV-2 salivary viral load.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of a total of 96 eligible participants enrolled from November 7, 2022, to January 19, 2023, 90 were accepted for the primary analysis. The use of 0.05% CPC mouthwash was not shown to be superior to placebo in change from baseline salivary Ct value at 30 min (difference vs. placebo, 0.640; 95% confidence interval [CI], -1.425 to 2.706; <jats:italic>P</jats:italic> = 0.543); 2 h (difference vs. placebo, 1.158; 95% CI, -0.797 to 3.112; <jats:italic>P</jats:italic> = 0.246); 4 h (difference vs. placebo, 1.283; 95% CI, -0.719 to 3.285; <jats:italic>P</jats:italic> = 0.209); 10 h (difference vs. placebo, 0.304; 95% CI, -1.777 to 2.385; <jats:italic>P</jats:italic> = 0.775); or 24 h (difference vs. placebo, 0.782; 95% CI, -1.195 to 2.759; <jats:italic>P</jats:italic> = 0.438). The use of 0.01% on-demand aqueous chlorine dioxide mouthwash was also not shown to be superior to placebo in change from baseline salivary Ct value at 30 min (difference vs. placebo, 0.905; 95% CI, -1.079 to 2.888; <jats:italic>P</jats:italic> = 0.371); 2 h (difference vs. placebo, 0.709; 95% CI, -1.275 to 2.693; <jats:italic>P</jats:italic> = 0.483); 4 h (difference vs. placebo, 0.220; 95% CI, -1.787 to 2.226; <jats:italic>P</jats:italic> = 0.830); 10 h (difference vs. placebo, 0.198; 95% CI, -1.901 to 2.296; <jats:italic>P</jats:italic> = 0.854); or 24 h (difference vs. placebo, 0.784; 95% CI, -1.236 to 2.804; <jats:italic>P</jats:italic> = 0.447).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>In asymptomatic to mildly symptomatic adults with COVID-19, compared to placebo, the use of 0.05% CPC and 0.01% on-demand aqueous chlorine dioxide mouthwash did not lead to a significant reduction in SARS-CoV-2 salivary viral load. Future studies of the efficacy of CPC and on-demand aqueous chlorine dioxide mouthwash on the viral viability of SARS-CoV-2 should be conducted using different specimen types and in multiple populations and settings.</jats:p>\n </jats:sec>",
"alternative-id": [
"4246"
],
"article-number": "491",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 December 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "10 April 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "25 April 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This open-label, randomized, placebo-controlled clinical trial was approved by the Ethical Committee of Osaka University (No. S22003) on 6/10/2022, and was registered with the Japan Registry of Clinical Trials (jRCT) (No. jRCTs051220107) on 18/10/2022. This trial was conducted in compliance with the provisions of the Declaration of Helsinki, Good Clinical Practice Guidelines, and local regulatory requirements. Patient registration was conducted from 7/11/2022 to 19/1/2023 in Osaka, Japan, and all of the participants provided written informed consent."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "S.K., D.O., S.T., and K.K. have received research support from Earth Corporation, Tokyo, Japan. H.M., H.Y., S.H., S.Y., R.M.S., and K.S. declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Onozuka",
"given": "Daisuke",
"sequence": "first"
},
{
"affiliation": [],
"family": "Takatera",
"given": "Satoko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsuo",
"given": "Hiroo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoshida",
"given": "Hisao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamaguchi",
"given": "Shigeto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamamoto",
"given": "Shungo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sada",
"given": "Ryuichi Minoda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suzuki",
"given": "Koichiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Konishi",
"given": "Keiji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kutsuna",
"given": "Satoshi",
"sequence": "additional"
}
],
"container-title": "BMC Oral Health",
"container-title-short": "BMC Oral Health",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
25
]
],
"date-time": "2024-04-25T04:01:44Z",
"timestamp": 1714017704000
},
"deposited": {
"date-parts": [
[
2024,
4,
25
]
],
"date-time": "2024-04-25T05:02:13Z",
"timestamp": 1714021333000
},
"funder": [
{
"name": "Earth Corporation, Tokyo, Japan"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
26
]
],
"date-time": "2024-04-26T00:27:07Z",
"timestamp": 1714091227258
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
4,
25
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
25
]
],
"date-time": "2024-04-25T00:00:00Z",
"timestamp": 1714003200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
25
]
],
"date-time": "2024-04-25T00:00:00Z",
"timestamp": 1714003200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12903-024-04246-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12903-024-04246-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12903-024-04246-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
4,
25
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
25
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2002032",
"author": "WJ Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "4246_CR1",
"unstructured": "Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.",
"volume": "382",
"year": "2020"
},
{
"key": "4246_CR2",
"unstructured": "World Health Organization. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. 2023. Available: https://covid19.who.int/. (Accessed 2023 6 December)."
},
{
"DOI": "10.1056/NEJMc2016359",
"author": "AL Wyllie",
"doi-asserted-by": "publisher",
"first-page": "1283",
"issue": "13",
"journal-title": "N Engl J Med",
"key": "4246_CR3",
"unstructured": "Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383(13):1283–6.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1177/0022034520918518",
"author": "J Xu",
"doi-asserted-by": "publisher",
"first-page": "989",
"issue": "8",
"journal-title": "J Dent Res",
"key": "4246_CR4",
"unstructured": "Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020;99(8):989.",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1038/s41591-021-01296-8",
"author": "N Huang",
"doi-asserted-by": "publisher",
"first-page": "892",
"issue": "5",
"journal-title": "Nat Med",
"key": "4246_CR5",
"unstructured": "Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892–903.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "249",
"issue": "2",
"journal-title": "Infect Dis Ther",
"key": "4246_CR6",
"unstructured": "Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash against Respiratory and oral tract pathogens. Infect Dis Ther. 2018;7(2):249–59.",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1038/s41598-022-18367-6",
"author": "R Takeda",
"doi-asserted-by": "publisher",
"first-page": "14050",
"issue": "1",
"journal-title": "Sci Rep",
"key": "4246_CR7",
"unstructured": "Takeda R, Sawa H, Sasaki M, Orba Y, Maishi N, Tsumita T, et al. Antiviral effect of cetylpyridinium chloride in mouthwash on SARS-CoV-2. Sci Rep. 2022;12(1):14050.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/jcm11061692",
"doi-asserted-by": "crossref",
"key": "4246_CR8",
"unstructured": "Garcia-Sanchez A, Pena-Cardelles JF, Ruiz S, Robles F, Ordonez-Fernandez E, Salgado-Peralvo AO et al. Efficacy of Pre-procedural mouthwashes against SARS-CoV-2: a systematic review of Randomized controlled trials. J Clin Med. 2022;11(6)."
},
{
"DOI": "10.1038/s41432-022-0253-z",
"doi-asserted-by": "crossref",
"key": "4246_CR9",
"unstructured": "Hernandez-Vasquez A, Barrenechea-Pulache A, Comande D, Azanedo D. Mouthrinses and SARS-CoV-2 viral load in saliva: a living systematic review. Evid Based Dent. 2022:1–7."
},
{
"DOI": "10.1016/j.sdentj.2022.01.006",
"author": "JPI Mezarina Mendoza",
"doi-asserted-by": "publisher",
"first-page": "167",
"issue": "3",
"journal-title": "Saudi Dent J",
"key": "4246_CR10",
"unstructured": "Mezarina Mendoza JPI, Trelles Ubillus BP, Salcedo Bolivar GT, Castaneda Palacios RDP, Herrera Lopez PSG, Padilla Rodriguez DA, Uchima Koecklin KH. Antiviral effect of mouthwashes against SARS-COV-2: a systematic review. Saudi Dent J. 2022;34(3):167–93.",
"volume": "34",
"year": "2022"
},
{
"DOI": "10.1016/j.jfma.2021.10.001",
"author": "MH Chen",
"doi-asserted-by": "publisher",
"first-page": "879",
"issue": "5",
"journal-title": "J Formos Med Assoc",
"key": "4246_CR11",
"unstructured": "Chen MH, Chang PC. The effectiveness of mouthwash against SARS-CoV-2 infection: a review of scientific and clinical evidence. J Formos Med Assoc. 2022;121(5):879–85.",
"volume": "121",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2022.2098059",
"author": "A Sanchez Barrueco",
"doi-asserted-by": "publisher",
"first-page": "1833",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "4246_CR12",
"unstructured": "Sanchez Barrueco A, Mateos-Moreno MV, Martinez-Beneyto Y, Garcia-Vazquez E, Campos Gonzalez A, Zapardiel Ferrero J, et al. Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial. Emerg Microbes Infect. 2022;11(1):1833–42.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1177/00220345231156415",
"author": "EL Bonn",
"doi-asserted-by": "publisher",
"first-page": "608",
"issue": "6",
"journal-title": "J Dent Res",
"key": "4246_CR13",
"unstructured": "Bonn EL, Rohrhofer A, Audebert FX, Lang H, Auer DL, Scholz KJ, et al. Efficacy of a Mouthwash Containing CHX and CPC in SARS-CoV-2-Positive patients: a Randomized Controlled Clinical Trial. J Dent Res. 2023;102(6):608–15.",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1177/00220345221102310",
"author": "A Alemany",
"doi-asserted-by": "publisher",
"first-page": "1450",
"issue": "12",
"journal-title": "J Dent Res",
"key": "4246_CR14",
"unstructured": "Alemany A, Perez-Zsolt D, Raich-Regue D, Munoz-Basagoiti J, Ouchi D, Laporte-Villar C, et al. Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: a double-blind Randomized Clinical Trial. J Dent Res. 2022;101(12):1450–6.",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"author": "MD Ferrer",
"doi-asserted-by": "publisher",
"first-page": "24392",
"issue": "1",
"journal-title": "Sci Rep",
"key": "4246_CR15",
"unstructured": "Ferrer MD, Barrueco AS, Martinez-Beneyto Y, Mateos-Moreno MV, Ausina-Marquez V, Garcia-Vazquez E, et al. Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2. Sci Rep. 2021;11(1):24392.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1111/jcpe.13905",
"author": "B Giulia",
"doi-asserted-by": "publisher",
"first-page": "158",
"issue": "2",
"journal-title": "J Clin Periodontol",
"key": "4246_CR16",
"unstructured": "Giulia B, Viktoria W, Robert K, Michael B, Nadine L, Jurgen B, et al. Eligibility and efficacy of a CPC- and CHX-based antiviral mouthwash for the elimination of SARS-CoV-2 from the saliva: a randomized, double-blind, controlled clinical trial. J Clin Periodontol. 2024;51(2):158–66.",
"volume": "51",
"year": "2024"
},
{
"DOI": "10.1016/j.mehy.2020.110436",
"author": "INR Reis",
"doi-asserted-by": "publisher",
"first-page": "110436",
"journal-title": "Med Hypotheses",
"key": "4246_CR17",
"unstructured": "Reis INR, do Amaral G, Mendoza AAH, das Gracas YT, Mendes-Correa MC, Romito GA, Pannuti CM. Can preprocedural mouthrinses reduce SARS-CoV-2 load in dental aerosols? Med Hypotheses. 2021;146:110436.",
"volume": "146",
"year": "2021"
},
{
"DOI": "10.1016/j.adaj.2019.06.024",
"author": "VC Marui",
"doi-asserted-by": "publisher",
"first-page": "1015",
"issue": "12",
"journal-title": "J Am Dent Assoc",
"key": "4246_CR18",
"unstructured": "Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. J Am Dent Assoc. 2019;150(12):1015–e261.",
"volume": "150",
"year": "2019"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"author": "CJ Seneviratne",
"doi-asserted-by": "publisher",
"first-page": "305",
"issue": "2",
"journal-title": "Infection",
"key": "4246_CR19",
"unstructured": "Seneviratne CJ, Balan P, Ko KKK, Udawatte NS, Lai D, Ng DHL, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305–11.",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1248/bpbreports.3.6_174",
"author": "T Shibata",
"doi-asserted-by": "publisher",
"first-page": "174",
"issue": "6",
"journal-title": "BPB Rep",
"key": "4246_CR20",
"unstructured": "Shibata T, Konishi K. The respiratory chain of Bacteria is a target of the disinfectant MA-T. BPB Rep. 2020;3(6):174–8.",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1248/bpbreports.4.3_78",
"author": "T Shibata",
"doi-asserted-by": "publisher",
"first-page": "78",
"issue": "3",
"journal-title": "BPB Rep",
"key": "4246_CR21",
"unstructured": "Shibata T, Urakawa R, Ono C, Akeda Y, Sakai T, Hamaguchi S, et al. Verification of MA-T Safety and Efficacy Against pathogens Including SARS-CoV-2. BPB Rep. 2021;4(3):78–84.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1248/bpb.b22-00046",
"author": "R Urakawa",
"doi-asserted-by": "publisher",
"first-page": "757",
"issue": "6",
"journal-title": "Biol Pharm Bull",
"key": "4246_CR22",
"unstructured": "Urakawa R, Shibata T, Sogou M, Takamori K, Inoue T, Konishi K, Sakai T. The Bactericidal Effect of MA-T for Factitiously contaminated and used masks. Biol Pharm Bull. 2022;45(6):757–62.",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1016/j.tox.2022.153254",
"author": "T Noguchi",
"doi-asserted-by": "publisher",
"first-page": "153254",
"journal-title": "Toxicology",
"key": "4246_CR23",
"unstructured": "Noguchi T, Tachibana K, Inoue T, Sakai T, Tsujikawa K, Fujio Y, et al. Safety evaluation of MA-T after ingestion in mice. Toxicology. 2022;477:153254.",
"volume": "477",
"year": "2022"
},
{
"key": "4246_CR24",
"unstructured": "Hologic. Aptima Multitest Swab Specimen Collection Kit. Hologic. 2022. Available: https://www.hologic.com/file/111046/download?token=VDYvcWpo. (Accessed 2024 26 January)."
},
{
"key": "4246_CR25",
"unstructured": "Hologic. Instructions for Using the Aptima® Multitest Swab Specimen Collection Kit for Patient-Collected Specimens. Hologic. 2022. Available: https://www.hologic.com/file/108196/download?token=bATaWv8L. (Accessed 2024 26 January)."
},
{
"key": "4246_CR26",
"unstructured": "TAKARA Bio Inc. Instructions for Using the Aptima® Multitest Swab Specimen Collection Kit for Patient-Collected Specimens, TAKARA Bio Inc. 2021. Available: https://catalog.takara-bio.co.jp/PDFS/rc346a_j.pdf. (Accessed 2024 26 January)."
},
{
"key": "4246_CR27",
"unstructured": "Hologic. SARS-CoV-2 Assay (Panther Fusion® System) Hologic. 2023. Available: https://www.fda.gov/media/138096/download. (Accessed 2024 26 January)."
},
{
"DOI": "10.1002/jmv.26749",
"author": "AK Cordes",
"doi-asserted-by": "publisher",
"first-page": "4438",
"issue": "7",
"journal-title": "J Med Virol",
"key": "4246_CR28",
"unstructured": "Cordes AK, Rehrauer WM, Accola MA, Wolk B, Hilfrich B, Heim A. Fully automated detection and differentiation of pandemic and endemic coronaviruses (NL63, 229E, HKU1, OC43 and SARS-CoV-2) on the hologic panther fusion. J Med Virol. 2021;93(7):4438–45.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26574",
"author": "RC Wong",
"doi-asserted-by": "publisher",
"first-page": "1226",
"issue": "3",
"journal-title": "J Med Virol",
"key": "4246_CR29",
"unstructured": "Wong RC, Wong AH, Ho YI, Leung EC, Lai RW. Performance evaluation of panther Fusion SARS-CoV-2 assay for detection of SARS-CoV-2 from deep throat saliva, nasopharyngeal, and lower-respiratory-tract specimens. J Med Virol. 2021;93(3):1226–8.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.jcv.2020.104580",
"author": "M Migueres",
"doi-asserted-by": "publisher",
"first-page": "104580",
"journal-title": "J Clin Virol",
"key": "4246_CR30",
"unstructured": "Migueres M, Mengelle C, Dimeglio C, Didier A, Alvarez M, Delobel P, et al. Saliva sampling for diagnosing SARS-CoV-2 infections in symptomatic patients and asymptomatic carriers. J Clin Virol. 2020;130:104580.",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1016/j.diagmicrobio.2021.115478",
"author": "M Migueres",
"doi-asserted-by": "publisher",
"first-page": "115478",
"issue": "3",
"journal-title": "Diagn Microbiol Infect Dis",
"key": "4246_CR31",
"unstructured": "Migueres M, Vellas C, Abravanel F, Da Silva I, Dimeglio C, Ferrer V, et al. Testing individual and pooled saliva samples for sars-cov-2 nucleic acid: a prospective study. Diagn Microbiol Infect Dis. 2021;101(3):115478.",
"volume": "101",
"year": "2021"
},
{
"author": "S-C Chow",
"edition": "3",
"key": "4246_CR32",
"unstructured": "Chow S-C, Shao J, Wang H, Lokhnygina Y. Sample size calculations in clinical research. 3rd ed. Boca Raton, FL: Chapman & Hall/CRC; 2018.",
"volume-title": "Sample size calculations in clinical research",
"year": "2018"
},
{
"DOI": "10.1002/9781118874905",
"doi-asserted-by": "crossref",
"key": "4246_CR33",
"unstructured": "Machin D, Campbell MJ, Tan SB, Tan SH. Sample sizes for clinical, laboratory and epidemiology studies. 4th ed. Wiley Blackwell; 2018."
},
{
"DOI": "10.1002/sim.1783",
"author": "SA Julious",
"doi-asserted-by": "publisher",
"first-page": "1921",
"issue": "12",
"journal-title": "Stat Med",
"key": "4246_CR34",
"unstructured": "Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23(12):1921–86.",
"volume": "23",
"year": "2004"
},
{
"DOI": "10.2307/2332010",
"author": "BL Welch",
"doi-asserted-by": "publisher",
"first-page": "350",
"issue": "3/4",
"journal-title": "Biometrika",
"key": "4246_CR35",
"unstructured": "Welch BL. The significance of the difference between two means when the population variances are unequal. Biometrika. 1938;29(3/4):350–62.",
"volume": "29",
"year": "1938"
},
{
"author": "JH Zar",
"edition": "2",
"key": "4246_CR36",
"unstructured": "Zar JH. Biostatistical Analysis. 2nd ed. Englewood Cliffs, New Jersey: Prentice-Hall; 1984.",
"volume-title": "Biostatistical Analysis",
"year": "1984"
},
{
"DOI": "10.1016/j.adaj.2021.05.021",
"author": "P Chaudhary",
"doi-asserted-by": "publisher",
"first-page": "903",
"issue": "11",
"journal-title": "J Am Dent Assoc",
"key": "4246_CR37",
"unstructured": "Chaudhary P, Melkonyan A, Meethil A, Saraswat S, Hall DL, Cottle J, et al. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: a randomized controlled trial. J Am Dent Assoc. 2021;152(11):903–8.",
"volume": "152",
"year": "2021"
},
{
"key": "4246_CR38",
"unstructured": "NCSS LLC. PASS 2023 Power Analysis and Sample Size Software. Kaysville, Utah. 2023. Available: www.ncss.com/software/pass."
},
{
"DOI": "10.1001/jama.2022.21506",
"author": "MJ Holmberg",
"doi-asserted-by": "publisher",
"first-page": "2155",
"issue": "21",
"journal-title": "JAMA",
"key": "4246_CR39",
"unstructured": "Holmberg MJ, Andersen LW. Adjustment for baseline characteristics in Randomized clinical trials. JAMA. 2022;328(21):2155–6.",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1186/s12903-023-03126-4",
"author": "T Ebrahimi",
"doi-asserted-by": "publisher",
"first-page": "443",
"issue": "1",
"journal-title": "BMC Oral Health",
"key": "4246_CR40",
"unstructured": "Ebrahimi T, Shamshiri AR, Alebouyeh M, Mohebbi SZ. Effectiveness of mouthwashes on reducing SARS-CoV-2 viral load in oral cavity: a systematic review and meta-analysis. BMC Oral Health. 2023;23(1):443.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1186/s12879-023-08669-z",
"author": "M Zhang",
"doi-asserted-by": "publisher",
"first-page": "678",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "4246_CR41",
"unstructured": "Zhang M, Meng N, Duo H, Yang Y, Dong Q, Gu J. Efficacy of mouthwash on reducing salivary SARS-CoV-2 viral load and clinical symptoms: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):678.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1080/20002297.2022.2030094",
"author": "M Bano-Polo",
"doi-asserted-by": "publisher",
"first-page": "2030094",
"issue": "1",
"journal-title": "J Oral Microbiol",
"key": "4246_CR42",
"unstructured": "Bano-Polo M, Martinez-Gil L, Sanchez Del Pino MM, Massoli A, Mingarro I, Leon R, Garcia-Murria MJ. Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles. J Oral Microbiol. 2022;14(1):2030094.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.virusres.2022.198791",
"author": "TL Meister",
"doi-asserted-by": "publisher",
"first-page": "198791",
"journal-title": "Virus Res",
"key": "4246_CR43",
"unstructured": "Meister TL, Gottsauner JM, Schmidt B, Heinen N, Todt D, Audebert F, et al. Mouthrinses against SARS-CoV-2 - high antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial. Virus Res. 2022;316:198791.",
"volume": "316",
"year": "2022"
},
{
"DOI": "10.1177/00220345211029269",
"author": "J Munoz-Basagoiti",
"doi-asserted-by": "publisher",
"first-page": "1265",
"issue": "11",
"journal-title": "J Dent Res",
"key": "4246_CR44",
"unstructured": "Munoz-Basagoiti J, Perez-Zsolt D, Leon R, Blanc V, Raich-Regue D, Cano-Sarabia M, et al. Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in Vitro. J Dent Res. 2021;100(11):1265–72.",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1186/s12903-022-02528-0",
"author": "AM Cardenas",
"doi-asserted-by": "publisher",
"first-page": "579",
"issue": "1",
"journal-title": "BMC Oral Health",
"key": "4246_CR45",
"unstructured": "Cardenas AM, Campos-Bijit V, Di Francesco F, Schwarz F, Cafferata EA, Vernal R. Electrolyzed water for the microbiologic control in the pandemic dental setting: a systematic review. BMC Oral Health. 2022;22(1):579.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.5888/pcd17.200266",
"author": "Z Brian",
"doi-asserted-by": "publisher",
"first-page": "E82",
"journal-title": "Prev Chronic Dis",
"key": "4246_CR46",
"unstructured": "Brian Z, Weintraub JA. Oral health and COVID-19: increasing the need for Prevention and Access. Prev Chronic Dis. 2020;17:E82.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1016/j.amjoto.2022.103549",
"author": "PJ Fantozzi",
"doi-asserted-by": "publisher",
"first-page": "103549",
"issue": "6",
"journal-title": "Am J Otolaryngol",
"key": "4246_CR47",
"unstructured": "Fantozzi PJ, Pampena E, Pierangeli A, Oliveto G, Sorrentino L, Di Vanna D, et al. Efficacy of antiseptic mouthrinses against SARS-CoV-2: a prospective randomized placebo-controlled pilot study. Am J Otolaryngol. 2022;43(6):103549.",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.3390/biomedicines11061694",
"doi-asserted-by": "crossref",
"key": "4246_CR48",
"unstructured": "Ting M, Dahlkemper A, Schwartz JJ, Woodfork M, Suzuki JB. Preprocedural viral load effects of oral antiseptics on SARS-CoV-2 in patients with COVID-19: a systematic review. Biomedicines. 2023;11(6)."
},
{
"author": "W Wang",
"first-page": "1843",
"issue": "18",
"journal-title": "JAMA",
"key": "4246_CR49",
"unstructured": "Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–4.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa377",
"author": "M Saito",
"doi-asserted-by": "publisher",
"first-page": "893",
"issue": "15",
"journal-title": "Clin Infect Dis",
"key": "4246_CR50",
"unstructured": "Saito M, Adachi E, Yamayoshi S, Koga M, Iwatsuki-Horimoto K, Kawaoka Y, Yotsuyanagi H. Gargle Lavage as a safe and sensitive alternative to Swab samples to Diagnose COVID-19: a Case Report in Japan. Clin Infect Dis. 2020;71(15):893–4.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1128/JCM.02427-20",
"doi-asserted-by": "crossref",
"key": "4246_CR51",
"unstructured": "Goldfarb DM, Tilley P, Al-Rawahi GN, Srigley JA, Ford G, Pedersen H et al. Self-collected saline Gargle Samples as an alternative to Health Care worker-collected nasopharyngeal swabs for COVID-19 diagnosis in outpatients. J Clin Microbiol. 2021;59(4)."
},
{
"DOI": "10.1007/s00216-022-03918-7",
"author": "BC Dhar",
"doi-asserted-by": "publisher",
"first-page": "2903",
"issue": "9",
"journal-title": "Anal Bioanal Chem",
"key": "4246_CR52",
"unstructured": "Dhar BC. Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic. Anal Bioanal Chem. 2022;414(9):2903–34.",
"volume": "414",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27145",
"author": "S Hiroi",
"doi-asserted-by": "publisher",
"first-page": "5917",
"issue": "10",
"journal-title": "J Med Virol",
"key": "4246_CR53",
"unstructured": "Hiroi S, Kubota-Koketsu R, Sasaki T, Morikawa S, Motomura K, Nakayama EE, et al. Infectivity assay for detection of SARS-CoV-2 in samples from patients with COVID-19. J Med Virol. 2021;93(10):5917–23.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(20)30237-1",
"author": "GM Joynt",
"doi-asserted-by": "publisher",
"first-page": "635",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "4246_CR54",
"unstructured": "Joynt GM, Wu WK, Understanding. COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020;20(6):635–6.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0242958",
"author": "I Arevalo-Rodriguez",
"doi-asserted-by": "publisher",
"first-page": "e0242958",
"issue": "12",
"journal-title": "PLoS ONE",
"key": "4246_CR55",
"unstructured": "Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, Zambrano-Achig P, Del Campo R, Ciapponi A, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS ONE. 2020;15(12):e0242958.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1038/s41598-023-35915-w",
"author": "S Yazawa",
"doi-asserted-by": "publisher",
"first-page": "8893",
"issue": "1",
"journal-title": "Sci Rep",
"key": "4246_CR56",
"unstructured": "Yazawa S, Yamazaki E, Saga Y, Itamochi M, Inasaki N, Shimada T, et al. Evaluation of SARS-CoV-2 isolation in cell culture from nasal/nasopharyngeal swabs or saliva specimens of patients with COVID-19. Sci Rep. 2023;13(1):8893.",
"volume": "13",
"year": "2023"
}
],
"reference-count": 56,
"references-count": 56,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcoralhealth.biomedcentral.com/articles/10.1186/s12903-024-04246-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Oral mouthwashes for asymptomatic to mildly symptomatic adults with COVID-19 and salivary viral load: a randomized, placebo-controlled, open-label clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}
