Lessons learned from the conduct of inpatient clinical trials in a pandemic
et al., Journal of Clinical and Translational Science, doi:10.1017/cts.2024.483, Oct 2024
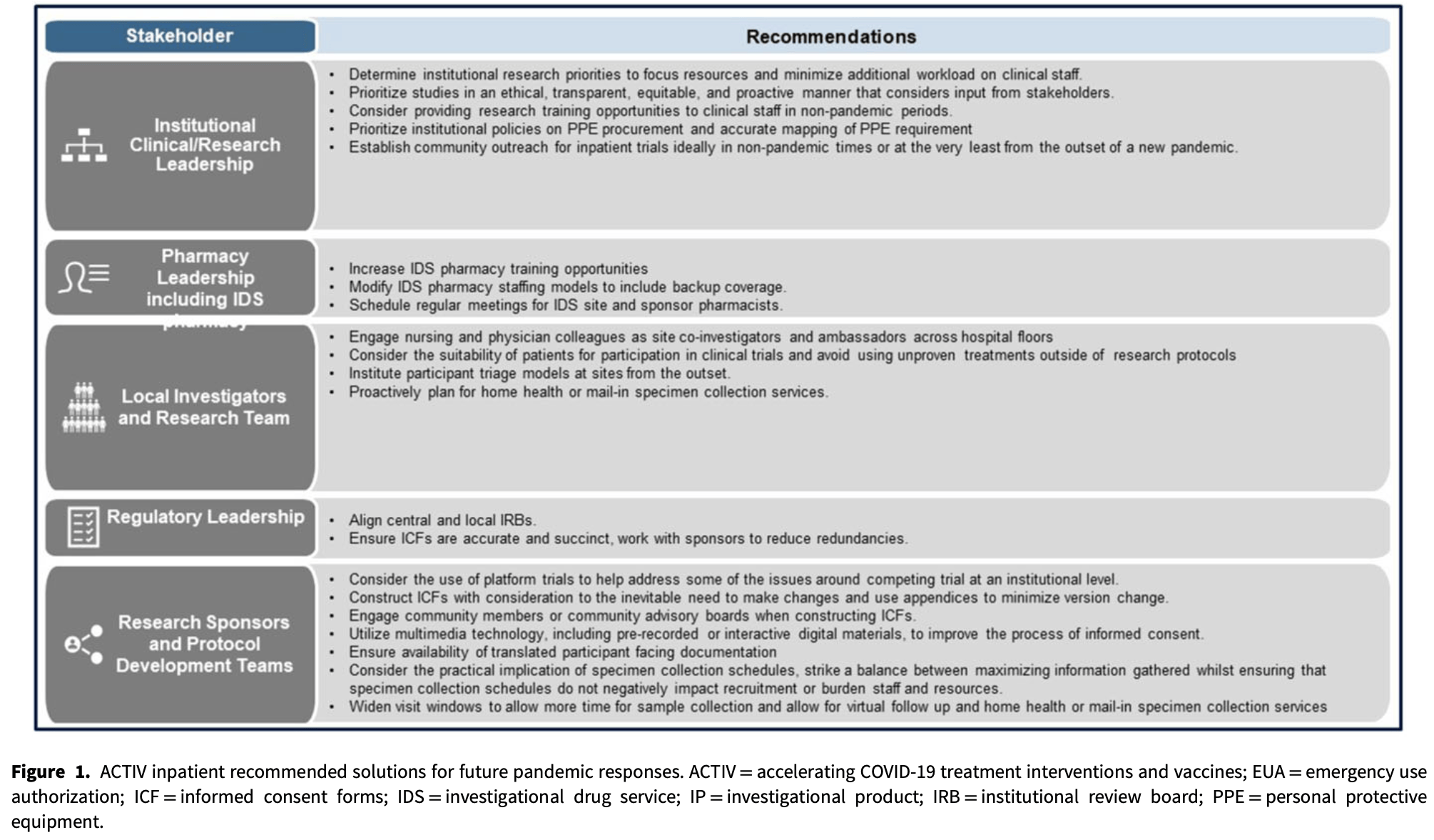
Review of challenges and potential solutions for conducting inpatient clinical trials during the pandemic, based on issues with the ACTIV trials. Challenges include competing clinical trials, interplay between clinical care and research conduct, infection control considerations, arduous consenting procedures, onerous trial procedures, and recruitment issues.
O’Halloran et al., 15 Oct 2024, peer-reviewed, 11 authors.
Contact: janeaohalloran@wustl.edu.
Lessons learned from the conduct of inpatient clinical trials in a pandemic
Journal of Clinical and Translational Science, doi:10.1017/cts.2024.483
Background: The COVID-19 pandemic amplified known challenges associated with the conduct of inpatient clinical trials, while also introducing new ones that needed to be addressed. Methods: Stakeholders based in the United States who participated in the conduct of inpatient therapeutic trials for the treatment of COVID-19 as part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines program identified challenges experienced in the conduct of these trials through a series of meeting to discuss and identify common themes. In addition, innovations developed to address these challenges and other potential solutions that may be utilized in future pandemics were highlighted. Results: Six thematic challenges including infection control considerations, the interplay between provision of clinical care and research, competing clinical trials, arduous consenting procedures, onerous procedural requirements, and participant recruitment including achieving representation of diverse populations were identified and are discussed here. Conclusions: Consideration of the lessons learned and recommendation outlined here may allow for more efficient conduct of inpatient clinical trials in future pandemics.
Author contributions. JAO, JRB, LKC, MKJ, AK, LHM, AM, MAO, SS, TY, and SUN all contributed to the conception of the article, collection of the lessons learned, interpretation of the information collected, and drafting and revising of the article. All authors were involved in the conduct of one or more ACTIV master protocol studies (ACTIV-1, -2, -3. -4. -5, or -6), which is where they drew the lessons learned collected. JAO takes responsibility for the article and will act as corresponding author. Funding statement. The authors did not receive funding support for the submitted work. The ACTIV clinical trials referenced in this article received funding from various United States Government funding agencies. Competing interests. Dr. Beitler reports prior consulting fees from Sedana Medical, Global Blood Therapeutics, Biomarck, and Arrowhead for work on advisory committees unrelated to this manuscript, funds from Sedana Medical paid to Columbia University for work as principal investigator of a clinical trial unrelated to this manuscript, and fees from Hamilton Medical for work as medical monitor of a clinical trial unrelated to this manuscript.
References
Angus, Optimizing the trade-off between learning and doing in a pandemic, JAMA, doi:10.1001/jama.2020.4984
Boulware, Corbie, Aguilar-Gaxiola, Combating structural inequities -diversity, equity, and inclusion in clinical and translational research, N Engl J Med, doi:10.1056/NEJMp2112233
Cohen, Rodgers, Contributing factors to personal protective equipment shortages during the COVID-19 pandemic, Prev Med, doi:10.1016/j.ypmed.2020.106263
Fisher, Kalbaugh, Challenging assumptions about minority participation in US clinical research, Am J Public Health, doi:10.2105/AJPH.2011.300279
Fitzsimmons, Idris, Pemu, Quantifying clinical trial diversity of FDA novel drug approvals, Ther Innov Regul Sci
Fogel, Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review, Contemp Clin Trials Commun
Gelinas, Lynch, Bierer, Cohen, When clinical trials compete: prioritising study recruitment, J Med Ethics
George, Duran, Norris, A systematic review of barriers and facilitators to minority research participation among African Americans, latinos, asian Americans, and pacific islanders, Am J Public Health, doi:10.2105/AJPH.2013.301706
Gordon, Micetich, Competing clinical trials in the same institution: ethical issues in subject selection and informed consent, IRB
Grant, Informed consent-We can and should do better, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.10848
Jain, Rollins, Jain, Access to Coronavirus disease 2019 clinical trials by English and non-English speakers is needed, Clin Infect Dis, doi:10.1093/cid/ciaa493
Lynch, Davitkov, Anderson, Infectious diseases society of America guidelines on infection prevention for healthcare personnel caring for patients with suspected or known COVID-19, Clin Infect Dis
North, Dougan, Sacks, Improving clinical trial enrollment -in the COVID-19 era and beyond, N Engl J Med
Ortega-Villa, Hynes, Levine, Evaluating demographic representation in clinical trials: use of the adaptive Coronavirus disease 2019 treatment trial (ACTT) as a test case, Open Forum Infect Dis
Paasche-Orlow, Taylor, Brancati, Readability standards for informed-consent forms as compared with actual readability, N Engl J Med
Rea, How serious is America's literacy problem?, Libr J
Sharp, Consent documents for oncology trials: does anybody read these things?, Am J Clin Oncol
Spector-Bagdady, Higgins, Aaronson, Coronavirus disease 2019 (COVID-19) clinical trial oversight at a major academic medical center: approach of Michigan medicine, Clin Infect Dis
Tikkanen, Woolhandler, Himmelstein, Hospital payer and racial/Ethnic mix at private academic medical centers in Boston and New York City, Int J Health Serv, doi:10.1177/0020731416689549
Turner, Steinberg, Weeks, Rodriguez, Cullen, Race/ ethnicity reporting and representation in US clinical trials: a cohort study, Lancet Reg Health Am, doi:10.1016/j.lana.2022.100252
DOI record:
{
"DOI": "10.1017/cts.2024.483",
"ISSN": [
"2059-8661"
],
"URL": "http://dx.doi.org/10.1017/cts.2024.483",
"abstract": "<jats:title>Abstract</jats:title>\n\t <jats:sec id=\"S2059866124004837_as1\">\n\t <jats:title>Background:</jats:title>\n\t <jats:p>The COVID-19 pandemic amplified known challenges associated with the conduct of inpatient clinical trials, while also introducing new ones that needed to be addressed.</jats:p>\n\t </jats:sec>\n\t <jats:sec id=\"S2059866124004837_as2\">\n\t <jats:title>Methods:</jats:title>\n\t <jats:p>Stakeholders based in the United States who participated in the conduct of inpatient therapeutic trials for the treatment of COVID-19 as part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines program identified challenges experienced in the conduct of these trials through a series of meeting to discuss and identify common themes. In addition, innovations developed to address these challenges and other potential solutions that may be utilized in future pandemics were highlighted.</jats:p>\n\t </jats:sec>\n\t <jats:sec id=\"S2059866124004837_as3\">\n\t <jats:title>Results:</jats:title>\n\t <jats:p>Six thematic challenges including infection control considerations, the interplay between provision of clinical care and research, competing clinical trials, arduous consenting procedures, onerous procedural requirements, and participant recruitment including achieving representation of diverse populations were identified and are discussed here.</jats:p>\n\t </jats:sec>\n\t <jats:sec id=\"S2059866124004837_as4\">\n\t <jats:title>Conclusions:</jats:title>\n\t <jats:p>Consideration of the lessons learned and recommendation outlined here may allow for more efficient conduct of inpatient clinical trials in future pandemics.</jats:p>\n\t </jats:sec>",
"alternative-id": [
"S2059866124004837"
],
"article-number": "e154",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8265-9471",
"affiliation": [],
"authenticated-orcid": false,
"family": "O’Halloran",
"given": "Jane A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Beitler",
"given": "Jeremy R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chung",
"given": "Lucy K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jain",
"given": "Mamta K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3091-632X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Khan",
"given": "Akram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Merck",
"given": "Lisa H.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3149-597X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mourad",
"given": "Ahmad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0008-3727-9611",
"affiliation": [],
"authenticated-orcid": false,
"family": "Oh",
"given": "Minn A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Shweta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yokum",
"given": "Tammy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nayak",
"given": "Seema U.",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical and Translational Science",
"container-title-short": "J. Clin. Trans. Sci.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
10,
15
]
],
"date-time": "2024-10-15T07:02:15Z",
"timestamp": 1728975735000
},
"deposited": {
"date-parts": [
[
2024,
10,
15
]
],
"date-time": "2024-10-15T07:02:21Z",
"timestamp": 1728975741000
},
"indexed": {
"date-parts": [
[
2024,
10,
16
]
],
"date-time": "2024-10-16T04:12:15Z",
"timestamp": 1729051935201
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 288,
"start": {
"date-parts": [
[
2024,
10,
15
]
],
"date-time": "2024-10-15T00:00:00Z",
"timestamp": 1728950400000
}
}
],
"link": [
{
"URL": "https://www.cambridge.org/core/services/aop-cambridge-core/content/view/S2059866124004837",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "56",
"original-title": [],
"prefix": "10.1017",
"published": {
"date-parts": [
[
2024
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
15
]
]
},
"published-print": {
"date-parts": [
[
2024
]
]
},
"publisher": "Cambridge University Press (CUP)",
"reference": [
{
"DOI": "10.1093/cid/ciaa560",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref1"
},
{
"DOI": "10.2307/3563649",
"article-title": "Competing clinical trials in the same institution: ethical issues in subject selection and informed consent",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "IRB",
"key": "S2059866124004837_ref5",
"volume": "24",
"year": "2002"
},
{
"key": "S2059866124004837_ref11",
"unstructured": "11. National Coalition for Literacy. Literacy and numeracy skills of U.S. adults. 2023. Available from https://www.piaacgateway.com/. Accessed January 3, 2024."
},
{
"DOI": "10.1136/medethics-2016-103680",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref2"
},
{
"DOI": "10.1093/cid/ciaa493",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref15"
},
{
"DOI": "10.1016/j.conctc.2018.08.001",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref16"
},
{
"article-title": "Infectious diseases society of America guidelines on infection prevention for healthcare personnel caring for patients with suspected or known COVID-19",
"author": "Lynch",
"journal-title": "Clin Infect Dis",
"key": "S2059866124004837_ref8",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.4984",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref6"
},
{
"DOI": "10.1093/ofid/ofad290",
"article-title": "Evaluating demographic representation in clinical trials: use of the adaptive Coronavirus disease 2019 treatment trial (ACTT) as a test case",
"author": "Ortega-Villa",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "S2059866124004837_ref23",
"volume": "10",
"year": "2023"
},
{
"key": "S2059866124004837_ref4",
"unstructured": "4. NIH National Center for Advancing Translational Sciences. Clinical and Translational Science Awards (CTSA) Program. Available from https://ncats.nih.gov/research/research-activities/ctsa. Accessed January 3, 2024."
},
{
"DOI": "10.2105/AJPH.2013.301706",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref20"
},
{
"DOI": "10.2105/AJPH.2011.300279",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref18"
},
{
"DOI": "10.1001/jamanetworkopen.2021.10848",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref9"
},
{
"article-title": "Race/ethnicity reporting and representation in US clinical trials: a cohort study",
"author": "Turner",
"first-page": "100252",
"journal-title": "Lancet Reg Health Am",
"key": "S2059866124004837_ref19",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1056/NEJMsa021212",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref10"
},
{
"DOI": "10.1007/s43441-023-00583-5",
"article-title": "Quantifying clinical trial diversity of FDA novel drug approvals",
"author": "Fitzsimmons",
"doi-asserted-by": "crossref",
"first-page": "175",
"journal-title": "Ther Innov Regul Sci",
"key": "S2059866124004837_ref17",
"volume": "58",
"year": "2024"
},
{
"key": "S2059866124004837_ref14",
"unstructured": "14. TextCompare. Flesch-Kincaid Grade Level Readability Calculator. 2023. Available from https://www.textcompare.org/readability/flesch-kincaid-grade-level/. Accessed January 3, 2024."
},
{
"DOI": "10.1016/j.ypmed.2020.106263",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref7"
},
{
"DOI": "10.1177/0020731416689549",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref21"
},
{
"DOI": "10.1056/NEJMp2019989",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref3"
},
{
"DOI": "10.1056/NEJMp2112233",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref22"
},
{
"article-title": "How serious is America’s literacy problem?",
"author": "Rea",
"journal-title": "Libr J",
"key": "S2059866124004837_ref12",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1097/01.coc.0000135925.83221.b3",
"doi-asserted-by": "publisher",
"key": "S2059866124004837_ref13"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cambridge.org/core/product/identifier/S2059866124004837/type/journal_article"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Lessons learned from the conduct of inpatient clinical trials in a pandemic",
"type": "journal-article",
"volume": "8"
}