Amiodarone for the Treatment of Arrhythmias in COVID-19 Patients Does Not Increase the Risk of Pulmonary Fibrosis: A Retrospective Cohort Study
et al., Cureus, doi:10.7759/cureus.34109, Jan 2023
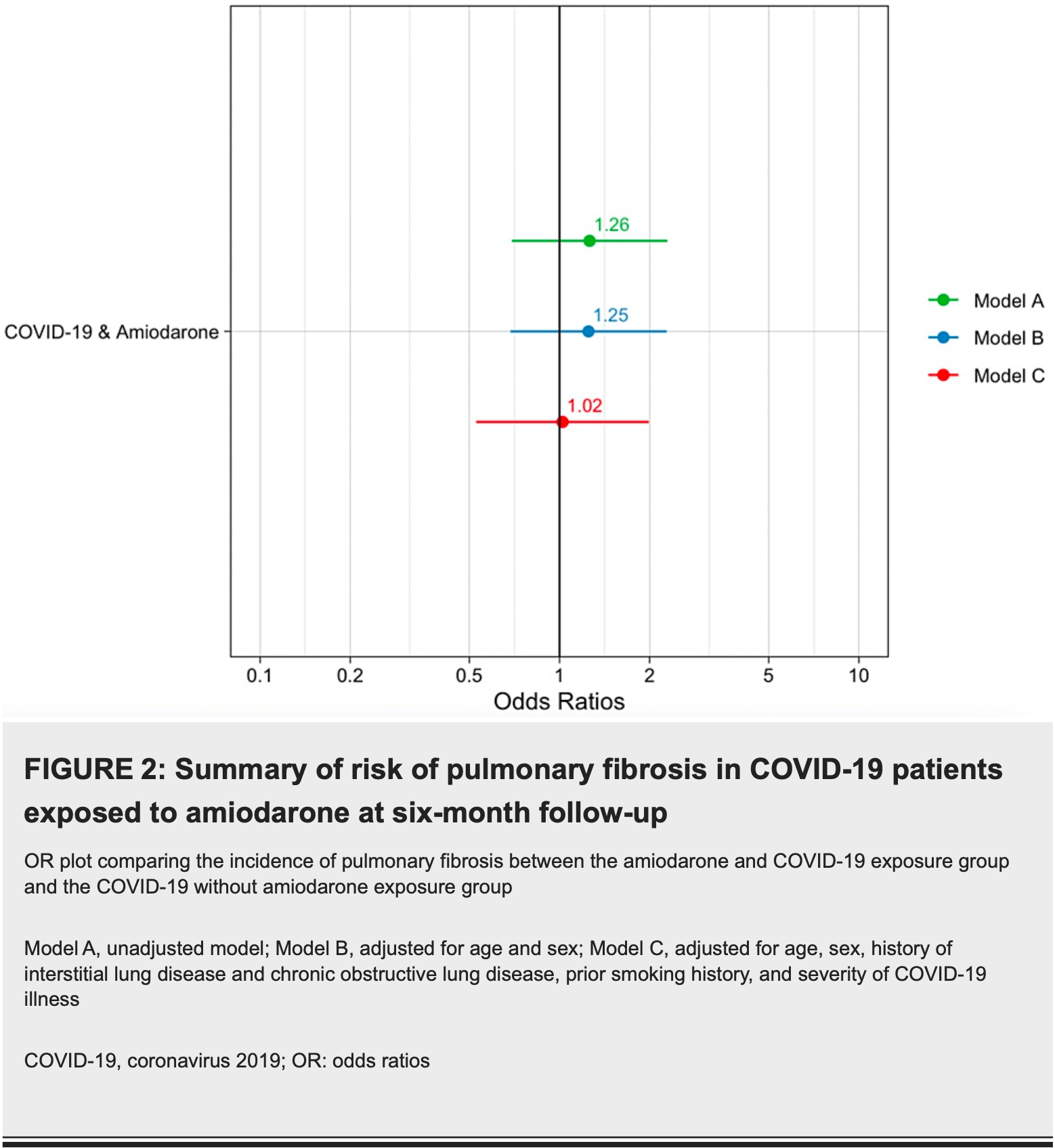
Retrospective 420 COVID-19 patients (210 with amiodarone exposure, 210 without) showing no significant difference in pulmonary fibrosis risk with amiodarone use.
Money et al., 23 Jan 2023, retrospective, USA, peer-reviewed, 7 authors, study period March 2020 - March 2022.
Contact: adamjcohen@usf.edu.
Amiodarone for the Treatment of Arrhythmias in COVID-19 Patients Does Not Increase the Risk of Pulmonary Fibrosis: A Retrospective Cohort Study
Cureus, doi:10.7759/cureus.34109
Amiodarone is a class III antiarrhythmic medication used to treat atrial and ventricular tachyarrhythmias. Pulmonary fibrosis from amiodarone use is a well-documented side effect. Pre-COVID-19 pandemic studies have shown that amiodarone-induced pulmonary fibrosis occurs in 1%-5% of patients and usually occurs between 12 to 60 months after initiation. The risk factors associated with amiodarone-induced pulmonary fibrosis include a high total cumulative dose (treatment longer than two months) and high maintenance dose (>400 mg/day). COVID-19 infection is also a known risk factor for developing pulmonary fibrosis and occurs in approximately 2%-6% of patients after a moderate illness. This study aims to assess the incidence of amiodarone in COVID-19 pulmonary fibrosis (ACPF). This is a retrospective cohort study with 420 patients with COVID-19 diagnoses between March 2020 and March 2022, comparing two populations, COVID-19 patients with exposure to amiodarone (N=210) and COVID-19 patients without amiodarone exposure (N=210). In our study, pulmonary fibrosis occurred in 12.9% of patients in the amiodarone exposure group compared to 10.5% of patients in the COVID-19 control group (p=0.543). In multivariate logistic analysis, which controlled for clinical covariates, amiodarone use in COVID-19 patients did not increase the odds of developing pulmonary fibrosis (odds ratio (OR): 1.02, 95% confidence interval (CI): 0.52-2.00). The clinical factors associated with the development of pulmonary fibrosis in both groups included a history of preexisting interstitial lung disease (ILD) (p=0.001), exposure to prior radiation therapy (p=0.021), and higher severity of COVID-19 illness (p<0.001). In conclusion, our study found no evidence that amiodarone use in COVID-19 patients increased the odds of developing pulmonary fibrosis at six-month follow-up. However, long-term amiodarone usage in the COVID-19 population should be based on the physician's discretion.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. The University of South Florida Institutional Review Board (IRB) Department issued approval STUDY003720. The IRB determined that this protocol meets the criteria for exemption from IRB review. In conducting this protocol, you are required to follow the requirements listed in the INVESTIGATOR MANUAL (HRP-103). Your study qualifies for a waiver of the requirement for signed authorization as outlined in the Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy Rule regulations at 45 CFR 164.512(i). A waiver of HIPAA authorization is granted for this retrospective chart review of patients who presented to Tampa General Hospital (TGH) or University of South Florida (USF) Outpatient Clinics and were diagnosed with COVID-19 within the date range in the protocol. This waiver allows the study team and/or its honest broker to obtain Protected Health Information (PHI) of the patients in this cohort from the TGH medical record (Epic) and the USF medical record (Epic) (ICD-10 codes: U07.1, T46.2X5). Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial..
References
Amin, Kakamad, Ahmed, Post COVID-19 pulmonary fibrosis; a meta-analysis study, Ann Med Surg (Lond), doi:10.1016/j.amsu.2022.103590
Bazdyrev, Rusina, Panova, Novikov, Grishagin et al., Lung fibrosis after COVID-19: treatment prospects, Pharmaceuticals, doi:10.3390/ph14080807
Boriani, Blomström-Lundqvist, Hohnloser, Safety and efficacy of dronedarone from clinical trials to real-world evidence: implications for its use in atrial fibrillation, Europace, doi:10.1093/europace/euz193
Cahoon, Flattery, Hess, Amiodarone: development, clinical indications, and safety, Prog Cardiovasc Nurs, doi:10.1111/j.0889-7204.2007.07398.x
Camus, Martin Wj 2nd, Rosenow, 3rd: Amiodarone pulmonary toxicity, Clin Chest Med, doi:10.1016/S0272-5231(03)00144-8
Castaldo, Aimo, Castiglione, Padalino, Emdin et al., Safety and efficacy of amiodarone in a patient with COVID-19, JACC Case Rep, doi:10.1016/j.jaccas.2020.04.053
Dusman, Stanton, Miles, Klein, Zipes et al., Clinical features of amiodarone-induced pulmonary toxicity, Circulation, doi:10.1161/01.cir.82.1.51
Gawałko, Kapłon-Cieślicka, Hohl, Dobrev, Linz, COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications, Int J Cardiol Heart Vasc, doi:10.1016/j.ijcha.2020.100631
George, Wells, Jenkins, Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy, Lancet Respir Med, doi:10.1016/S2213-2600(20)30225-3
Jackevicius, Tom, Essebag, Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone, Am J Cardiol, doi:10.1016/j.amjcard.2011.04.024
January, Wann, Calkins, AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons, Circulation, doi:10.1161/CIR.0000000000000665
Jessurun, Crijns, Amiodarone pulmonary toxicity, BMJ, doi:10.1136/bmj.314.7081.619
Kosseifi, Halawa, Bailey, Micklewright, Roy et al., Reduction of amiodarone pulmonary toxicity in patients treated with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, Ther Adv Respir Dis, doi:10.1177/1753465809348015
Marian, Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries, Cardiovasc Pathol, doi:10.1016/j.carpath.2020.107278
Martin, Mechanisms of amiodarone pulmonary toxicity, Clin Chest Med, doi:10.1016/S0272-5231(21)00677-8
Martin, Rosenow EC 3rd: Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part I), Chest, doi:10.1378/chest.93.5.1067
Myoung, Two years of COVID-19 pandemic: where are we now?, J Microbiol, doi:10.1007/s12275-022-1679-x
Papiris, Triantafillidou, Kolilekas, Markoulaki, Manali, Amiodarone: review of pulmonary effects and toxicity, Drug Saf, doi:10.2165/11532320-000000000-00000
Schwaiblmair, Berghaus, Haeckel, Wagner, Scheidt, Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect?, Clin Res Cardiol, doi:10.1007/s00392-010-0181-3
Vassallo, Trohman, Prescribing amiodarone: an evidence-based review of clinical indications, JAMA, doi:10.1001/jama.298.11.1312
Wang, Kream, Stefano, Long-term respiratory and neurological sequelae of COVID-19, Med Sci Monit, doi:10.12659/MSM.928996
Wolkove, Baltzan, Amiodarone pulmonary toxicity, Can Respir J, doi:10.1155/2009/282540
Wyse, Waldo, Dimarco, A comparison of rate control and rhythm control in patients with atrial fibrillation, N Engl J Med, doi:10.1056/NEJMoa021328
DOI record:
{
"DOI": "10.7759/cureus.34109",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.34109",
"author": [
{
"affiliation": [],
"family": "Money",
"given": "David B",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lee",
"given": "Dae Hyun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hadar",
"given": "Ari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doherty",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malanga",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Serino",
"given": "Alexa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Adam J",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
1,
23
]
],
"date-time": "2023-01-23T23:35:56Z",
"timestamp": 1674516956000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T19:57:24Z",
"timestamp": 1707508644000
},
"indexed": {
"date-parts": [
[
2024,
2,
10
]
],
"date-time": "2024-02-10T00:28:47Z",
"timestamp": 1707524927108
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
23
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/133667-amiodarone-for-the-treatment-of-arrhythmias-in-covid-19-patients-does-not-increase-the-risk-of-pulmonary-fibrosis-a-retrospective-cohort-study",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2023,
1,
23
]
]
},
"published-print": {
"date-parts": [
[
2023,
1,
23
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1161/01.cir.82.1.51",
"article-title": "Clinical features of amiodarone-induced pulmonary toxicity",
"author": "Dusman RE",
"doi-asserted-by": "publisher",
"journal-title": "Circulation",
"key": "ref1",
"unstructured": "Dusman RE, Stanton MS, Miles WM, Klein LS, Zipes DP, Fineberg NS, Heger JJ. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990, 82:51-9. 10.1161/01.cir.82.1.51",
"volume": "82",
"year": "1990"
},
{
"DOI": "10.1161/CIR.0000000000000665",
"article-title": "2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons",
"author": "January CT",
"doi-asserted-by": "publisher",
"journal-title": "Circulation",
"key": "ref2",
"unstructured": "January CT, Wann LS, Calkins H, et al.. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019, 140:e125-51. 10.1161/CIR.0000000000000665",
"volume": "140",
"year": "2019"
},
{
"DOI": "10.1001/jama.298.11.1312",
"article-title": "Prescribing amiodarone: an evidence-based review of clinical indications",
"author": "Vassallo P",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref3",
"unstructured": "Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA. 2007, 298:1312-22. 10.1001/jama.298.11.1312",
"volume": "298",
"year": "2007"
},
{
"DOI": "10.1177/1753465809348015",
"article-title": "Reduction of amiodarone pulmonary toxicity in patients treated with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers",
"author": "Kosseifi SG",
"doi-asserted-by": "publisher",
"journal-title": "Ther Adv Respir Dis",
"key": "ref4",
"unstructured": "Kosseifi SG, Halawa A, Bailey B, Micklewright M, Roy TM, Byrd RP Jr. Reduction of amiodarone pulmonary toxicity in patients treated with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ther Adv Respir Dis. 2009, 3:289-94. 10.1177/1753465809348015",
"volume": "3",
"year": "2009"
},
{
"article-title": "Mechanisms of amiodarone pulmonary toxicity",
"author": "Martin WJ 2nd",
"journal-title": "Clin Chest Med",
"key": "ref5",
"unstructured": "Martin WJ 2nd. Mechanisms of amiodarone pulmonary toxicity. Clin Chest Med. 1990, 11:131-8.",
"volume": "11",
"year": "1990"
},
{
"DOI": "10.1136/bmj.314.7081.619",
"article-title": "Amiodarone pulmonary toxicity",
"author": "Jessurun GA",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "ref6",
"unstructured": "Jessurun GA, Crijns HJ. Amiodarone pulmonary toxicity. BMJ. 1997, 314:619-20. 10.1136/bmj.314.7081.619",
"volume": "314",
"year": "1997"
},
{
"DOI": "10.1016/S0272-5231(03)00144-8",
"article-title": "Amiodarone pulmonary toxicity",
"author": "Camus P",
"doi-asserted-by": "publisher",
"journal-title": "Clin Chest Med",
"key": "ref7",
"unstructured": "Camus P, Martin WJ 2nd, Rosenow EC 3rd. Amiodarone pulmonary toxicity. Clin Chest Med. 2004, 25:65-75. 10.1016/S0272-5231(03)00144-8",
"volume": "25",
"year": "2004"
},
{
"DOI": "10.1111/j.0889-7204.2007.07398.x",
"article-title": "Amiodarone: development, clinical indications, and safety",
"author": "Cahoon W Jr",
"doi-asserted-by": "publisher",
"journal-title": "Prog Cardiovasc Nurs",
"key": "ref8",
"unstructured": "Cahoon W Jr, Flattery MP, Hess ML. Amiodarone: development, clinical indications, and safety. Prog Cardiovasc Nurs. 2007, 22:173-6. 10.1111/j.0889-7204.2007.07398.x",
"volume": "22",
"year": "2007"
},
{
"DOI": "10.1093/europace/euz193",
"article-title": "Safety and efficacy of dronedarone from clinical trials to real-world evidence: implications for its use in atrial fibrillation",
"author": "Boriani G",
"doi-asserted-by": "publisher",
"journal-title": "Europace",
"key": "ref9",
"unstructured": "Boriani G, Blomström-Lundqvist C, Hohnloser SH, et al.. Safety and efficacy of dronedarone from clinical trials to real-world evidence: implications for its use in atrial fibrillation. Europace. 2019, 21:1764-75. 10.1093/europace/euz193",
"volume": "21",
"year": "2019"
},
{
"DOI": "10.1155/2009/282540",
"article-title": "Amiodarone pulmonary toxicity",
"author": "Wolkove N",
"doi-asserted-by": "publisher",
"journal-title": "Can Respir J",
"key": "ref10",
"unstructured": "Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009, 16:43-8. 10.1155/2009/282540",
"volume": "16",
"year": "2009"
},
{
"DOI": "10.1378/chest.93.5.1067",
"article-title": "Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part I)",
"author": "Martin WJ 2nd",
"doi-asserted-by": "publisher",
"journal-title": "Chest",
"key": "ref11",
"unstructured": "Martin WJ 2nd, Rosenow EC 3rd. Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part I). Chest. 1988, 93:1067-75. 10.1378/chest.93.5.1067",
"volume": "93",
"year": "1988"
},
{
"DOI": "10.1056/NEJMoa021328",
"article-title": "A comparison of rate control and rhythm control in patients with atrial fibrillation",
"author": "Wyse DG",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref12",
"unstructured": "Wyse DG, Waldo AL, DiMarco JP, et al.. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002, 347:1825-33. 10.1056/NEJMoa021328",
"volume": "347",
"year": "2002"
},
{
"DOI": "10.1016/S2213-2600(20)30225-3",
"article-title": "Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy",
"author": "George PM",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "ref13",
"unstructured": "George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020, 8:807-15. 10.1016/S2213-2600(20)30225-3",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.12659/MSM.928996",
"article-title": "Long-term respiratory and neurological sequelae of COVID-19",
"author": "Wang F",
"doi-asserted-by": "publisher",
"journal-title": "Med Sci Monit",
"key": "ref14",
"unstructured": "Wang F, Kream RM, Stefano GB. Long-term respiratory and neurological sequelae of COVID-19. Med Sci Monit. 2020, 26:e928996. 10.12659/MSM.928996",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.jaccas.2020.04.053",
"article-title": "Safety and efficacy of amiodarone in a patient with COVID-19",
"author": "Castaldo N",
"doi-asserted-by": "publisher",
"journal-title": "JACC Case Rep",
"key": "ref15",
"unstructured": "Castaldo N, Aimo A, Castiglione V, Padalino C, Emdin M, Tascini C. Safety and efficacy of amiodarone in a patient with COVID-19. JACC Case Rep. 2020, 2:1307-10. 10.1016/j.jaccas.2020.04.053",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.carpath.2020.107278",
"article-title": "Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries",
"author": "Marian AJ",
"doi-asserted-by": "publisher",
"journal-title": "Cardiovasc Pathol",
"key": "ref16",
"unstructured": "Marian AJ. Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries. Cardiovasc Pathol. 2021, 50:107278. 10.1016/j.carpath.2020.107278",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1007/s00392-010-0181-3",
"article-title": "Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect?",
"author": "Schwaiblmair M",
"doi-asserted-by": "publisher",
"journal-title": "Clin Res Cardiol",
"key": "ref17",
"unstructured": "Schwaiblmair M, Berghaus T, Haeckel T, Wagner T, von Scheidt W. Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect?. Clin Res Cardiol. 2010, 99:693-700. 10.1007/s00392-010-0181-3",
"volume": "99",
"year": "2010"
},
{
"DOI": "10.2165/11532320-000000000-00000",
"article-title": "Amiodarone: review of pulmonary effects and toxicity",
"author": "Papiris SA",
"doi-asserted-by": "publisher",
"journal-title": "Drug Saf",
"key": "ref18",
"unstructured": "Papiris SA, Triantafillidou C, Kolilekas L, Markoulaki D, Manali ED. Amiodarone: review of pulmonary effects and toxicity. Drug Saf. 2010, 33:539-58. 10.2165/11532320-000000000-00000",
"volume": "33",
"year": "2010"
},
{
"DOI": "10.1016/j.amjcard.2011.04.024",
"article-title": "Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone",
"author": "Jackevicius CA",
"doi-asserted-by": "publisher",
"journal-title": "Am J Cardiol",
"key": "ref19",
"unstructured": "Jackevicius CA, Tom A, Essebag V, et al.. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011, 108:705-10. 10.1016/j.amjcard.2011.04.024",
"volume": "108",
"year": "2011"
},
{
"DOI": "10.1007/s12275-022-1679-x",
"article-title": "Two years of COVID-19 pandemic: where are we now?",
"author": "Myoung J",
"doi-asserted-by": "publisher",
"journal-title": "J Microbiol",
"key": "ref20",
"unstructured": "Myoung J. Two years of COVID-19 pandemic: where are we now?. J Microbiol. 2022, 60:235-7. 10.1007/s12275-022-1679-x",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.1016/j.amsu.2022.103590",
"article-title": "Post COVID-19 pulmonary fibrosis; a meta-analysis study",
"author": "Hama Amin BJ",
"doi-asserted-by": "publisher",
"journal-title": "Ann Med Surg (Lond)",
"key": "ref21",
"unstructured": "Hama Amin BJ, Kakamad FH, Ahmed GS, et al.. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond). 2022, 77:103590. 10.1016/j.amsu.2022.103590",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1016/j.ijcha.2020.100631",
"article-title": "COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications",
"author": "Gawałko M",
"doi-asserted-by": "publisher",
"journal-title": "Int J Cardiol Heart Vasc",
"key": "ref22",
"unstructured": "Gawałko M, Kapłon-Cieślicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020, 30:100631. 10.1016/j.ijcha.2020.100631",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.3390/ph14080807",
"article-title": "Lung fibrosis after COVID-19: treatment prospects",
"author": "Bazdyrev E",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceuticals (Basel)",
"key": "ref23",
"unstructured": "Bazdyrev E, Rusina P, Panova M, Novikov F, Grishagin I, Nebolsin V. Lung fibrosis after COVID-19: treatment prospects. Pharmaceuticals (Basel). 2021, 14:10.3390/ph14080807",
"volume": "14",
"year": "2021"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/133667-amiodarone-for-the-treatment-of-arrhythmias-in-covid-19-patients-does-not-increase-the-risk-of-pulmonary-fibrosis-a-retrospective-cohort-study"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Amiodarone for the Treatment of Arrhythmias in COVID-19 Patients Does Not Increase the Risk of Pulmonary Fibrosis: A Retrospective Cohort Study",
"type": "journal-article"
}