Effect of background therapy with Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents on COVID-19 outcomes
et al., medRxiv, doi:10.1101/2024.12.07.24318645, Dec 2024
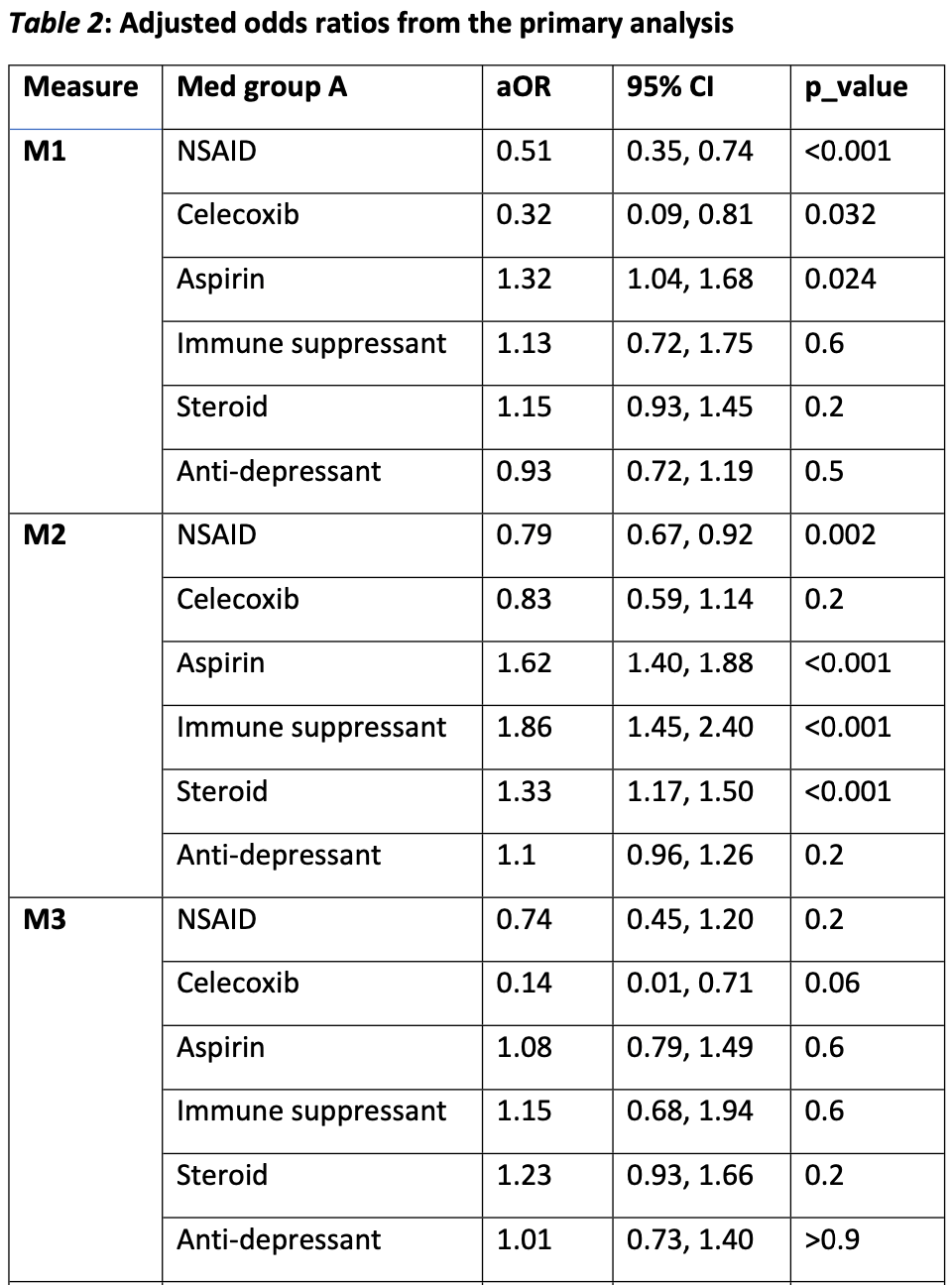
Retrospective 485,779 osteoarthritis patients in the US showing lower mortality with non-aspirin NSAIDs and celecoxib, and higher mortality with aspirin. Aspirin was associated with higher hospitalization in COVID-positive and COVID-negative patients. Comparison of the COVID-positive and COVID-negative results suggests significant residual confounding.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
substantial unadjusted confounding by indication possible.
Study covers aspirin, ibuprofen, and indomethacin.
|
risk of death, 32.0% higher, OR 1.32, p = 0.02, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Miele et al., 8 Dec 2024, retrospective, USA, preprint, 12 authors, study period 1 January, 2020 - 31 March, 2021.
Contact: lmiele@lsuhsc.edu.
Effect of background therapy with Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents on COVID-19 outcomes
doi:10.1101/2024.12.07.24318645
Background: Inflammation plays a complex, incompletely understood role in the pathogenesis of acute COVID-19 and Post-Acute Sequelae of SARS-CoV-2 infection (PASC or "Long COVID"). Systemic acute inflammation resulting in cytokine storm, hypercoagulability and endothelial damage is thought to be a central mechanism for severe morbidity and mortality in acute COVID-19. Anti-inflammatory medications taken routinely for chronic conditions prior to contracting COVID-19 ("background medications") may modulate acute COVID-19 outcomes. Methods: Using data from the National COVID Cohort Collaborative (N3C) enclave, we estimated effects of six classes of background medications on acute COVID outcomes. Medication classes included aspirin, celecoxib, other NSAIDS, steroids, immune suppressants, and antidepressants. Acute COVID outcomes included probability of hospital admission, inpatient mortality, and mortality among diagnosed COVID patients. Each medication class was compared to benzodiazepines (excluding midazolam) which served as a comparator/control. Only adult COVID patients with pre-existing osteoarthritis and without any diagnosed autoimmune disease were included in the analyses. Random effects logistic regression models were used to adjust for covariates and data contributing organization. Medication effects also were estimated for COVID-negative cases. Results: Non-aspirin NSAIDS were associated with lower mortality among diagnosed COVID-19 patients: adjusted Odds Ratio (aOR)=0.32 (p=.032) for celecoxib; aOR=0.51 (p<.001) for NSAIDS other than aspirin and celecoxib. For inpatient mortality: aOR=0.34 (p=.060) for celecoxib and aOR=0.74 (p=.200) for other non-aspirin NSAIDS. Similar effects were observed for COVID-negative cases, including for inpatient mortality: aOR=0.21 (p<.001) for celecoxib and .
References
Abdelhaleem, Kassab, El-Nassan, Khalil, Recent advances in the development of celecoxib analogs as anticancer agents: A review, Arch Pharm (Weinheim), doi:10.1002/ardp.202200326
Baghaki, Yalcin, Baghaki, Aydin, Daghan et al., COX2 inhibition in the treatment of COVID-19: Review of literature to propose repositioning of celecoxib for randomized controlled studies, Int J Infect Dis, doi:10.1016/j.ijid.2020.09.1466
Bahmani, Chegini, Ghanbari, Sheykhsaran, Aghbash et al., Severe acute respiratory syndrome coronavirus 2 infection: Role of interleukin-6 and the inflammatory cascade, World J Virol, doi:10.5501/wjv.v11.i3.113
Boroujeni, Sekrecka, Antonczyk, Hassani, Sekrecki et al., Dysregulated Interferon Response and Immune Hyperactivation in Severe COVID-19: Targeting STATs as a Novel Therapeutic Strategy, Front Immunol, doi:10.3389/fimmu.2022.888897
Borton, Bhangoo, Quah, Stephen, Howard, Aspirin monotherapy is a suitable standard thromboprophylactic agent following total hip arthroplasty, Hip Int, doi:10.1177/1120700021990544
Christopher, Chute, Hopkins University ; Anzalone, Manna, Saha et al., Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C, Front Pharmacol, doi:10.3389/fphar.2022.899198
Danziger-Isakov, Blumberg, Manuel, Sester, Impact of COVID-19 in solid organ transplant recipients, Am J Transplant, doi:10.1111/ajt.16449
El-Malah, Gineinah, Deb, Khayyat, Bansal et al., Selective COX-2 Inhibitors: Road from Success to Controversy and the Quest for Repurposing, Pharmaceuticals, doi:10.3390/ph15070827
Eteraf-Oskouei, Najafi, The relationship between the serotonergic system and COVID-19 disease: A review, Heliyon, doi:10.1016/j.heliyon.2022.e09544
Eyitemi, Thomas, Ramos, Feng, Ezekwesili, SARS-CoV-2: Review of Conditions Associated With Severe Disease and Mortality, Int J Prev Med, doi:10.4103/ijpvm.IJPVM_640_20
Gadi, Shetty, Potential of Anti-inflammatory Molecules in the Chemoprevention of Breast Cancer, Recent Adv Inflamm Allergy Drug Discov, doi:10.2174/2772270816666220829090716
Ghaznavi, Mohammadghasemipour, Shirvaliloo, Momeni, Metanat et al., Short-term celecoxib (celebrex) adjuvant therapy: a clinical trial study on COVID-19 patients, Inflammopharmacology, doi:10.1007/s10787-022-01029-4
Gimeno, Mestres-Truyol, Ojeda-Montes, Macip, Saldivar-Espinoza et al., Prediction of Novel Inhibitors of the Main Protease (M-pro) of SARS-CoV-2 through Consensus Docking and Drug Reposition, Int J Mol Sci, doi:10.3390/ijms21113793
Haendel, Chute, Bennett, The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment, J Am Med Inform Assoc, doi:10.1093/jamia/ocaa196
Hsieh, Wang, Chen, Zhao, Savitz et al., Drug repurposing for COVID-19 using graph neural network and harmonizing multiple evidence, Sci Rep, doi:10.1038/s41598-021-02353-5
Ipema, Brand, Deb, Dev, Unl, Antiplatelet and anticoagulation therapy after revascularization for lower extremity artery disease: a national survey and literature overview, J Cardiovasc Surg, doi:10.23736/S0021-9509.20.11402-2
Jin, Qian, Lin, Liu, Cyclooxygenase-2-Prostaglandin E2 pathway: A key player in tumor-associated immune cells, Front Oncol, doi:10.3389/fonc.2023.1099811
Kalinski, Regulation of immune responses by prostaglandin E2, J Immunol, doi:10.4049/jimmunol.1101029
Liao, Identification of potential new COVID-19 treatments via RWD-driven drug repurposing, Sci Rep, doi:10.1038/s41598-023-40033-8
Liu, Qu, Yan, Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity, Cancer Cell Int, doi:10.1186/s12935-015-0260-7
Micallef, Soeiro, Ap, French Society of Pharmacology T. Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection, Therapie, doi:10.1016/j.therap.2020.05.003
Montazersaheb, Khatibi, Hejazi, Tarhriz, Farjami et al., COVID-19 infection: an overview on cytokine storm and related interventions, Virol J, doi:10.1186/s12985-022-01814-1
Newell, Waickman, Inflammation, immunity, and antigen persistence in postacute sequelae of SARS-CoV-2 infection, Curr Opin Immunol, doi:10.1016/j.coi.2022.102228
Newman, Muscat, Potential Role of Non-Steroidal Anti-Inflammatory Drugs in Colorectal Cancer Chemoprevention for Inflammatory Bowel Disease: An Umbrella Review, Cancers, doi:10.3390/cancers15041102
Nso, Nassar, Zirkiyeva, Mbome, Ngonge et al., Factors Impacting Stent Thrombosis in Patients With Percutaneous Coronary Intervention and Coronary Stenting: A Systematic Review and Meta-Analysis, Cureus, doi:10.7759/cureus.23973
Obeid, Libby, Husni, Wang, Wisniewski et al., Cardiorenal risk of celecoxib compared with naproxen or ibuprofen in arthritis patients: insights from the PRECISION trial, Eur Heart J Cardiovasc Pharmacother, doi:10.1093/ehjcvp/pvac015
Patrono, Cyclooxygenase Inhibitors and Cancer: The Missing Pieces, J Pharmacol Exp Ther, doi:10.1124/jpet.122.001631
Quan-Charlson Paper Here: Quan, Li, Couris, Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries, Am J Epidemiol, doi:10.1093/aje/kwq433
Rashid, Khan, Elsori, Rehman, Tanzeelah et al., Non-steroidal antiinflammatory drugs and biomarkers: A new paradigm in colorectal cancer, Front Med, doi:10.3389/fmed.2023.1130710
Reese, Coleman, Chan, Blau, Callahan et al., NSAID use and clinical outcomes in COVID-19 patients: a 38-center retrospective cohort study, Virol J, doi:10.1186/s12985-022-01813-2
Ujjawal, Gupta, Ghosh, Jain, Bandyopadhyay et al., Aspirin for Primary Prevention of Coronary Artery Disease, Curr Probl Cardiol, doi:10.1016/j.cpcardiol.2020.100553
Vasudeva, Challa, Rifai, Polana, Duran et al., Prevalence of cardiovascular diseases in COVID-19 related mortality in the United States, Prog Cardiovasc Dis, doi:10.1016/j.pcad.2022.09.002
Whoreafc, Sterne, Murthy, Diaz, Slutsky et al., Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis, JAMA, doi:10.1001/jama.2020.17023
Ye, Li, Li, Song, Sun et al., The Efficacy and Safety of Celecoxib in Addition to Standard Cancer Therapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Curr Oncol, doi:10.3390/curroncol29090482
Zhang, Qin, Fei, Shen, Zhou et al., Anti-inflammatory and immune therapy in severe coronavirus disease 2019 (COVID-19) patients: An update, Clin Immunol, doi:10.1016/j.clim.2022.109022
DOI record:
{
"DOI": "10.1101/2024.12.07.24318645",
"URL": "http://dx.doi.org/10.1101/2024.12.07.24318645",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Inflammation plays a complex, incompletely understood role in the pathogenesis of acute COVID-19 and Post-Acute Sequelae of SARS-CoV-2 infection (PASC or “Long COVID”). Systemic acute inflammation resulting in cytokine storm, hypercoagulability and endothelial damage is thought to be a central mechanism for severe morbidity and mortality in acute COVID-19. Anti-inflammatory medications taken routinely for chronic conditions prior to contracting COVID-19 (“background medications”) may modulate acute COVID-19 outcomes.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Using data from the National COVID Cohort Collaborative (N3C) enclave, we estimated effects of six classes of background medications on acute COVID outcomes. Medication classes included aspirin, celecoxib, other NSAIDS, steroids, immune suppressants, and antidepressants. Acute COVID outcomes included probability of hospital admission, inpatient mortality, and mortality among diagnosed COVID patients. Each medication class was compared to benzodiazepines (excluding midazolam) which served as a comparator/control. Only adult COVID patients with pre-existing osteoarthritis and without any diagnosed autoimmune disease were included in the analyses. Random effects logistic regression models were used to adjust for covariates and data contributing organization. Medication effects also were estimated for COVID-negative cases.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Non-aspirin NSAIDS were associated with lower mortality among diagnosed COVID-19 patients: adjusted Odds Ratio (aOR)=0.32 (p=.032) for celecoxib; aOR=0.51 (p<.001) for NSAIDS other than aspirin and celecoxib. For inpatient mortality: aOR=0.34 (p=.060) for celecoxib and aOR=0.74 (p=.200) for other non-aspirin NSAIDS. Similar effects were observed for COVID-negative cases, including for inpatient mortality: aOR=0.21 (p<.001) for celecoxib and aOR=0.34 (p<.001) for other non-aspirin NSAIDS. Secondary analyses examined alternative explanations for results.</jats:p></jats:sec><jats:sec><jats:title>Discussion</jats:title><jats:p>Protective effects were observed for non-aspirin NSAIDS, especially celecoxib. However, those estimated effects implicitly assume the medication classes did not differ on the probability a true COVID-19 case was diagnosed. The similarity of COVID-positive and COVID-negative results suggest possible missing covariates. However, such similarity plausibly could stem from a medication having both “direct” and “indirect” effects on COVID outcomes. Adjudicating among the alternative interpretations would require data beyond those available. However, the effects observed for non-aspirin NSAIDS, while possibly biased, rationalize further investigation using study designs constructed to overcome the limitations of existing datasets.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2024,
12,
8
]
]
},
"author": [
{
"ORCID": "https://orcid.org/0000-0002-5853-7287",
"affiliation": [],
"authenticated-orcid": false,
"family": "Miele",
"given": "Lucio",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0003-3058-824X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chu",
"given": "San",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hillegass",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jurkovitz",
"given": "Claudine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beasley",
"given": "William",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4140-459X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chen",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anzalone",
"given": "A Jerrod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fort",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kirwan",
"given": "John",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1999-4351",
"affiliation": [],
"authenticated-orcid": false,
"family": "Melancon",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hodder",
"given": "Sally",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Horswell",
"given": "Ronald",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
12,
9
]
],
"date-time": "2024-12-09T05:05:45Z",
"timestamp": 1733720745000
},
"deposited": {
"date-parts": [
[
2024,
12,
10
]
],
"date-time": "2024-12-10T12:10:20Z",
"timestamp": 1733832620000
},
"group-title": "Public and Global Health",
"indexed": {
"date-parts": [
[
2024,
12,
11
]
],
"date-time": "2024-12-11T16:11:07Z",
"timestamp": 1733933467579,
"version": "3.30.2"
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
12,
8
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
8
]
],
"date-time": "2024-12-08T00:00:00Z",
"timestamp": 1733616000000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2024.12.07.24318645",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2024,
12,
8
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2024,
12,
8
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.3389/fphar.2022.899198",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.1"
},
{
"DOI": "10.1186/s12985-022-01814-1",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.2"
},
{
"DOI": "10.3389/fimmu.2022.888897",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.3"
},
{
"DOI": "10.5501/wjv.v11.i3.113",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.4"
},
{
"DOI": "10.1016/j.coi.2022.102228",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.5"
},
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.6"
},
{
"DOI": "10.1016/j.clim.2022.109022",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.7"
},
{
"DOI": "10.1016/j.therap.2020.05.003",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.8"
},
{
"DOI": "10.1016/j.heliyon.2022.e09544",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.9"
},
{
"DOI": "10.1093/jamia/ocaa196",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.10"
},
{
"DOI": "10.1186/s12985-022-01813-2",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.11"
},
{
"DOI": "10.1093/aje/kwq433",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.12"
},
{
"DOI": "10.1111/ajt.16449",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.13"
},
{
"DOI": "10.7759/cureus.23973",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.14"
},
{
"DOI": "10.1177/1120700021990544",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.15"
},
{
"DOI": "10.23736/S0021-9509.20.11402-2",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.16"
},
{
"DOI": "10.1016/j.cpcardiol.2020.100553",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.17"
},
{
"DOI": "10.1016/j.pcad.2022.09.002",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.18"
},
{
"DOI": "10.1016/j.ijid.2020.09.1466",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.19"
},
{
"DOI": "10.1007/s10787-022-01029-4",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.20"
},
{
"DOI": "10.1038/s41598-023-40033-8",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.21"
},
{
"DOI": "10.1038/s41598-021-02353-5",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.22"
},
{
"DOI": "10.3390/ijms21113793",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.23"
},
{
"DOI": "10.4049/jimmunol.1101029",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.24"
},
{
"DOI": "10.1093/ehjcvp/pvac015",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.25"
},
{
"DOI": "10.1186/s12935-015-0260-7",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.26"
},
{
"DOI": "10.3389/fonc.2023.1099811",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.27"
},
{
"DOI": "10.3390/curroncol29090482",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.28"
},
{
"DOI": "10.1124/jpet.122.001631",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.29"
},
{
"DOI": "10.3389/fmed.2023.1130710",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.30"
},
{
"DOI": "10.3390/cancers15041102",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.31"
},
{
"DOI": "10.2174/2772270816666220829090716",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.32"
},
{
"DOI": "10.1002/ardp.202200326",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.33"
},
{
"DOI": "10.3390/ph15070827",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.34"
},
{
"DOI": "10.4103/ijpvm.IJPVM_640_20",
"doi-asserted-by": "publisher",
"key": "2024121004101106000_2024.12.07.24318645v1.35"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2024.12.07.24318645"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Effect of background therapy with Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents on COVID-19 outcomes",
"type": "posted-content"
}