The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID-19 infection
et al., The FEBS Journal, doi:10.1111/febs.15784, Feb 2021
Retrospective 10,477 patients in Israel, showing lower risk of COVID-19 cases with existing aspiring use.
|
risk of case, 27.6% lower, RR 0.72, p = 0.04, treatment 73 of 1,621 (4.5%), control 589 of 8,856 (6.7%), NNT 47, adjusted per study, odds ratio converted to relative risk.
|
|
risk of death, 62.4% lower, RR 0.38, p = 0.51, treatment 1 of 21 (4.8%), control 6 of 91 (6.6%), adjusted per study, odds ratio converted to relative risk.
|
|
time to viral-, 9.6% lower, relative time 0.90, p = 0.045, treatment 73, control 589, time to 2nd negative test.
|
|
time to viral-, 14.8% lower, relative time 0.85, p = 0.005, treatment 73, control 589, time to 1st negative test.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Merzon et al., 23 Feb 2021, retrospective, Israel, peer-reviewed, 8 authors.
The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID‐19 infection
The FEBS Journal, doi:10.1111/febs.15784
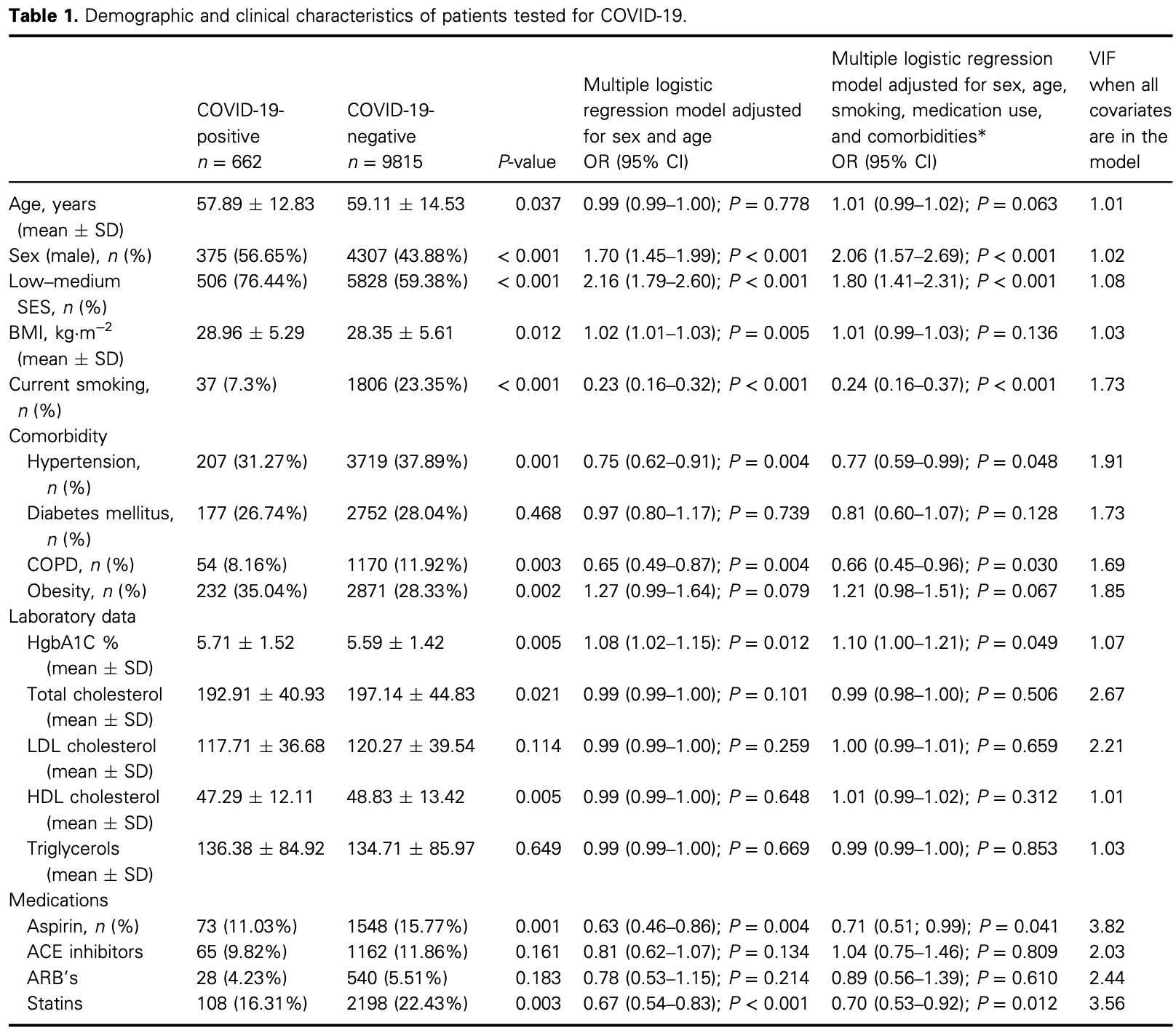
Acetylsalicylic acid (aspirin) is commonly used for primary and secondary prevention of cardiovascular diseases. Aspirin use is associated with better outcomes among COVID-19 positive patients. We hypothesized that the aspirin use for primary cardiovascular disease prevention might have a protective effect on COVID-19 susceptibility and disease duration. We conducted a retrospective population-based cross-sectional study, utilizing data from the Leumit Health Services database. The proportion of patients treated with aspirin was significantly lower among the COVID-19-positive group, as compared to the COVID-19-negative group [73 (11.03%) vs. 1548 (15.77%); P = 0.001]. Aspirin use was associated with lower likelihood of COVID-19 infection, as compared to nonusers (adjusted OR 0.71 (95% CI, 0.52 to 0.99; P = 0.041). Aspirin users were older (68.06 AE 12.79 vs. 56.63 AE 12.28 years of age; P < 0.001), presented a lower BMI (28.77 AE 5.4 vs. 30.37 AE 4.55; P < 0.0189), and showed higher prevalence of hypertension (56, 76.71%), diabetes (47, 64.38%), and COPD (11, 15.07%) than the aspirin nonusers (151, 25.64%, P < 0.001; 130, 22.07%, P < 0.001; and 43, 7.3%, P = 0.023, respectively). Moreover, COVID-19 disease duration (considered as the time between the first positive and second negative COVID-19 RT-PCR test results) among aspirin users was significantly shorter, as compared to aspirin nonusers (19.8 AE 7.8 vs. 21.9 AE 7.9 P = 0.045). Among hospitalized COVID-positive patients, a higher proportion of surviving subjects were treated with aspirin (20, 19.05%), as opposed to 1 dead subject (14.29%), although this difference was not significant (P = 0.449). In conclusion, we observed an inverse association between the likelihood of COVID-19 infection, disease duration and mortality, and aspirin use for primary prevention.
Abbreviations COVID-19, coronavirus SARS-CoV-2; CVD, cardiovascular disease; LHS, Leumit Health Services; SES, socioeconomic status; STING, stimulator of interferon genes.
Conflict of interest The authors declare no conflict of interest.
Author contributions EM and ElM have designed the study, EM, IG, SV, AGC, MF-M, and ElM have analyzed the data, AG and MF-M have produced figures, EM has supervised the study, and all authors have written the manuscript.
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/febs.15784.
References
Arnett, Blumenthal, Albert, Buroker, Goldberger et al., ACC/ AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines, J Am Coll Cardiol
Berthelot, Drouet, Lioté, Kawasakilike diseases and thrombotic coagulopathy in COVID-19: delayed over-activation of the STING pathway?, Emerg Microbes Infect
Berthelot, Lioté, COVID-19 as a STING disorder with delayed over-secretion of interferon-beta, EBioMedicine
Boutaud, Sosa, Amin, Oram, Adler et al., Inhibition of the biosynthesis of prostaglandin E2 by low-dose aspirin: implications for adenocarcinoma metastasis, Cancer Prev Res (Phila)
Chang, Mo, Yuan, Tao, Peng et al., Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection, Am J Respir Crit Care Med
Chodick, Heymann, Shalev, Kookia, The epidemiology of diabetes in a large Israeli HMO, Eur J Epidemiol
Chow, Khanna, Kethireddy, Yamane, Levine et al., Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19, Anesth Analg, doi:10.1213/ANE.0000000000005292
Coulombe, Jaworska, Verway, Tzelepis, Massoud et al., Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages, Immunity
Coulombe, Jaworska, Verway, Tzelepis, Massoud et al., Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages, Immunity
De Wit, Van Doremalen, Falzarano, Munster, SARS and MERS: recent insights into emerging coronaviruses, Nat Rev Microbiol
Desborough, Keeling, The aspirin story -from willow to wonder drug, Br J Haematol
Gaziano, Brotons, Coppolecchia, Cricelli, Darius et al., Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial, Lancet
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy, JAMA
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Hussain, Javeed, Ashraf, Zhao, Mukhtar et al., Aspirin and immune system, Int Immunopharmacol
Kim, Multicollinearity and misleading statistical results, Korean J Anesthesiol
Lazear, Schoggins, Diamond, Shared and distinct functions of type I and type III interferons, Immunity
Manne, Denorme, Middleton, Portier, Rowley et al., Platelet gene expression and function in patients with COVID-19, Blood
Mcneil, Nelson, Woods, Lockery, Wolfe et al., Effect of aspirin on all-cause mortality in the healthy elderly, N Engl J Med
Nakhaei, Hiscott, Lin, STING-ing the antiviral pathway, J Mol Cell Biol
Patrono, Baigent, Role of aspirin in primary prevention of cardiovascular disease, Nat Rev Cardiol
Pringle, Ward, Chilvers, Assessment of the completeness and accuracy of computer medical records in four practices committed to recording data on computer, Br J Gen Pract
Rennert, Peterburg, Prevalence of selected chronic diseases in Israel, Isr Med Assoc J
Rennert, Peterburg, Prevalence of selected chronic diseases in Israel, Isr Med Assoc J
Seyedpour, Khodaei, Loghman, Seyedpour, Kisomi et al., Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: a systematic review of in vitro and in vivo studies, J Cell Physiol
Shen, Yi, Sun, Bi, Du et al., Proteomic and metabolomic characterization of COVID-19 patient sera, Cell
Sriram, Insel, A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance, Br J Pharmacol
Subir, Jagat, Kalyan, Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19), Diabetes Metab Syndr
Vankadari, Wilce, Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26, Emerg Microbes Infect
Violi, Pastori, Cangemi, Pignatelli, Loffredo, Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge, Thromb Haemost
Wickham, Grolemund, R for Data Science: Import, Tidy, Transform, Visualize, and Model Data
Wu, Zhao, Yu, Chen, Song et al., A new coronavirus associated with human respiratory disease in China, Nature
Zaid, Puhm, Allaeys, Naya, Oudghiri et al., Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19, Circ Res
Zhang, Liu, Wang, Yang, Li et al., SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19, J Hematol Oncol
Üller, Mair, Saalm, Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study, Influenza Other Respir Viruses
DOI record:
{
"DOI": "10.1111/febs.15784",
"ISSN": [
"1742-464X",
"1742-4658"
],
"URL": "http://dx.doi.org/10.1111/febs.15784",
"abstract": "<jats:p>Acetylsalicylic acid (aspirin) is commonly used for primary and secondary prevention of cardiovascular diseases. Aspirin use is associated with better outcomes among COVID‐19 positive patients. We hypothesized that the aspirin use for primary cardiovascular disease prevention might have a protective effect on COVID‐19 susceptibility and disease duration. We conducted a retrospective population‐based cross‐sectional study, utilizing data from the Leumit Health Services database. The proportion of patients treated with aspirin was significantly lower among the COVID‐19‐positive group, as compared to the COVID‐19‐negative group [73 (11.03%) vs. 1548 (15.77%); <jats:italic>P</jats:italic> = 0.001]. Aspirin use was associated with lower likelihood of COVID‐19 infection, as compared to nonusers (adjusted OR 0.71 (95% CI, 0.52 to 0.99; <jats:italic>P</jats:italic> = 0.041). Aspirin users were older (68.06 ± 12.79 vs. 56.63 ± 12.28 years of age; <jats:italic>P</jats:italic> < 0.001), presented a lower BMI (28.77 ± 5.4 vs. 30.37 ± 4.55; <jats:italic>P</jats:italic> < 0.0189), and showed higher prevalence of hypertension (56, 76.71%), diabetes (47, 64.38%), and COPD (11, 15.07%) than the aspirin nonusers (151, 25.64%, <jats:italic>P</jats:italic> < 0.001; 130, 22.07%, <jats:italic>P</jats:italic> < 0.001; and 43, 7.3%, <jats:italic>P</jats:italic> = 0.023, respectively). Moreover, COVID‐19 disease duration (considered as the time between the first positive and second negative COVID‐19 RT–PCR test results) among aspirin users was significantly shorter, as compared to aspirin nonusers (19.8 ± 7.8 vs. 21.9 ± 7.9 <jats:italic>P</jats:italic> = 0.045). Among hospitalized COVID‐positive patients, a higher proportion of surviving subjects were treated with aspirin (20, 19.05%), as opposed to 1 dead subject (14.29%), although this difference was not significant (<jats:italic>P</jats:italic> = 0.449). In conclusion, we observed an inverse association between the likelihood of COVID‐19 infection, disease duration and mortality, and aspirin use for primary prevention.</jats:p>",
"alternative-id": [
"10.1111/febs.15784"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-11-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-02-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-04-19"
}
],
"author": [
{
"affiliation": [
{
"name": "Leumit Health Services Tel‐Aviv Israel"
},
{
"name": "Department of Family Medicine Sackler Faculty of Medicine Tel‐Aviv University Israel"
}
],
"family": "Merzon",
"given": "Eugene",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Leumit Health Services Tel‐Aviv Israel"
},
{
"name": "Department of Family Medicine Sackler Faculty of Medicine Tel‐Aviv University Israel"
}
],
"family": "Green",
"given": "Ilan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Leumit Health Services Tel‐Aviv Israel"
},
{
"name": "Department of Family Medicine Sackler Faculty of Medicine Tel‐Aviv University Israel"
}
],
"family": "Vinker",
"given": "Shlomo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Leumit Health Services Tel‐Aviv Israel"
},
{
"name": "Department of Family Medicine Sackler Faculty of Medicine Tel‐Aviv University Israel"
}
],
"family": "Golan‐Cohen",
"given": "Avivit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Azrieli Faculty of Medicine Bar‐Ilan University Safed Israel"
}
],
"family": "Gorohovski",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Leumit Health Services Tel‐Aviv Israel"
},
{
"name": "Department of Management Bar‐Ilan University Safed Israel"
}
],
"family": "Avramovich",
"given": "Eva",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0329-4599",
"affiliation": [
{
"name": "Azrieli Faculty of Medicine Bar‐Ilan University Safed Israel"
}
],
"authenticated-orcid": false,
"family": "Frenkel‐Morgenstern",
"given": "Milana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Leumit Health Services Tel‐Aviv Israel"
},
{
"name": "Medicine C Department Clinical Immunology and Allergy Division Barzilai University Medical Center Ben‐Gurion University of the Negev Ashkelon Israel"
}
],
"family": "Magen",
"given": "Eli",
"sequence": "additional"
}
],
"container-title": "The FEBS Journal",
"container-title-short": "The FEBS Journal",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"febs.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
23
]
],
"date-time": "2021-02-23T21:01:16Z",
"timestamp": 1614114076000
},
"deposited": {
"date-parts": [
[
2023,
8,
28
]
],
"date-time": "2023-08-28T06:40:08Z",
"timestamp": 1693204808000
},
"indexed": {
"date-parts": [
[
2024,
3,
24
]
],
"date-time": "2024-03-24T07:34:08Z",
"timestamp": 1711265648205
},
"is-referenced-by-count": 34,
"issue": "17",
"issued": {
"date-parts": [
[
2021,
4,
19
]
]
},
"journal-issue": {
"issue": "17",
"published-print": {
"date-parts": [
[
2021,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
19
]
],
"date-time": "2021-04-19T00:00:00Z",
"timestamp": 1618790400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/febs.15784",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/febs.15784",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://febs.onlinelibrary.wiley.com/doi/pdf/10.1111/febs.15784",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "5179-5189",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2021,
4,
19
]
]
},
"published-online": {
"date-parts": [
[
2021,
4,
19
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1001/jama.2020.5394",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1111/bjh.14520",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.1016/S0140-6736(18)31924-X",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1056/NEJMoa1803955",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1016/j.jacc.2019.03.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1038/s41569-019-0225-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/j.intimp.2011.11.021",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"article-title": "Assessment of the completeness and accuracy of computer medical records in four practices committed to recording data on computer",
"author": "Pringle M",
"first-page": "537",
"journal-title": "Br J Gen Pract",
"key": "e_1_2_10_10_1",
"volume": "45",
"year": "1995"
},
{
"article-title": "Prevalence of selected chronic diseases in Israel",
"author": "Rennert G",
"first-page": "404",
"journal-title": "Isr Med Assoc J",
"key": "e_1_2_10_11_1",
"volume": "3",
"year": "2001"
},
{
"article-title": "Prevalence of selected chronic diseases in Israel",
"author": "Rennert G",
"first-page": "404",
"journal-title": "Isr Med Assoc J",
"key": "e_1_2_10_12_1",
"volume": "3",
"year": "2001"
},
{
"DOI": "10.1023/B:EJEP.0000006635.36802.c8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"key": "e_1_2_10_14_1",
"unstructured": "https://www.health.gov.il/Subjects/disease/corona/Documents/bz‐259818720.pdf"
},
{
"key": "e_1_2_10_15_1",
"unstructured": "World Health Organization (WHO)(2020)Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Interim guidance.https://apps.who.int/iris/bitstream/handle/10665/331501/WHO‐COVID‐19‐laboratory‐2020.5‐eng.pdf?sequence¼1&isAllowed¼y"
},
{
"DOI": "10.1111/irv.12421",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1016/j.immuni.2019.03.025",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1038/nrmicro.2016.81",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1016/j.immuni.2014.02.013",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1158/1940-6207.CAPR-16-0094",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1016/j.immuni.2014.02.013",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1038/s41586-020-2008-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1016/j.dsx.2020.07.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1093/jmcb/mjp048",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1016/j.ebiom.2020.102801",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.1080/22221751.2020.1785336",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1111/bph.15082",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1213/ANE.0000000000005292",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1182/blood.2020007214",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317703",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_1"
},
{
"DOI": "10.1016/j.cell.2020.05.032",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"DOI": "10.1186/s13045-020-00954-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"DOI": "10.1002/jcp.30032",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_1"
},
{
"DOI": "10.1080/22221751.2020.1739565",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_34_1"
},
{
"DOI": "10.4097/kja.19087",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_35_1"
},
{
"key": "e_1_2_10_36_1",
"unstructured": "Guidance for discharge and ending of isolation of people with COVID‐19.https://www.ecdc.europa.eu/sites/default/files/documents/Guidance‐for‐discharge‐and‐ending‐of‐isolation‐of‐people‐with‐COVID‐19.pdf"
},
{
"DOI": "10.1164/rccm.202003-0524LE",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_1"
},
{
"DOI": "10.1055/s-0040-1710317",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_1"
},
{
"key": "e_1_2_10_39_1",
"unstructured": "https://clinicaltrials.gov/ct2/show/results/NCT04381936"
},
{
"key": "e_1_2_10_40_1",
"unstructured": "https://govextra.gov.il/media/17976/coronavirus_med_guidelines.pdf"
},
{
"key": "e_1_2_10_41_1",
"unstructured": "WickhamH&GrolemundG(2016)R for Data Science: Import Tidy Transform Visualize and Model Data O'Reilly Media."
}
],
"reference-count": 40,
"references-count": 40,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.1111/FEBS.15784/v1/review1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/FEBS.15784/v1/decision1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/FEBS.15784/v2/decision1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/FEBS.15784/v1/review2",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/FEBS.15784/v2/response1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/FEBS.15784/v2/review1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.15784"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cell Biology",
"Molecular Biology",
"Biochemistry"
],
"subtitle": [],
"title": "The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID‐19 infection",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "288"
}