Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: treatment modalities, clinical response, and outcomes
et al., Annals of Intensive Care, doi:10.1186/s13613-023-01150-9, Jun 2023
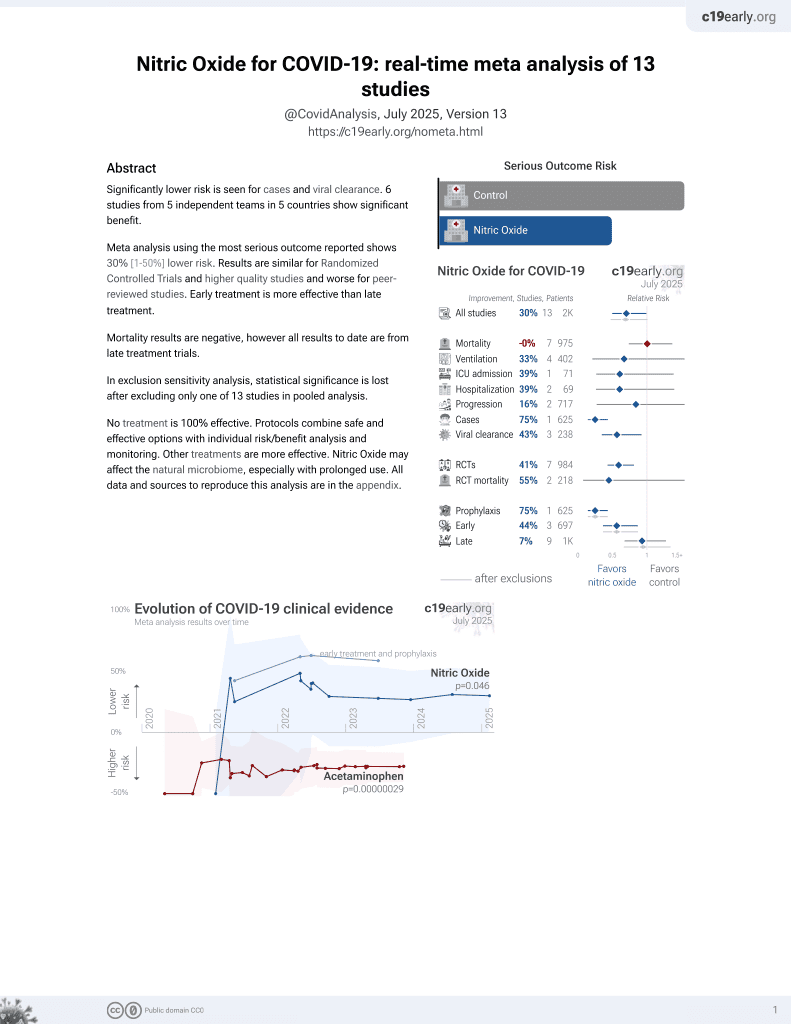
43rd treatment shown to reduce risk in
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
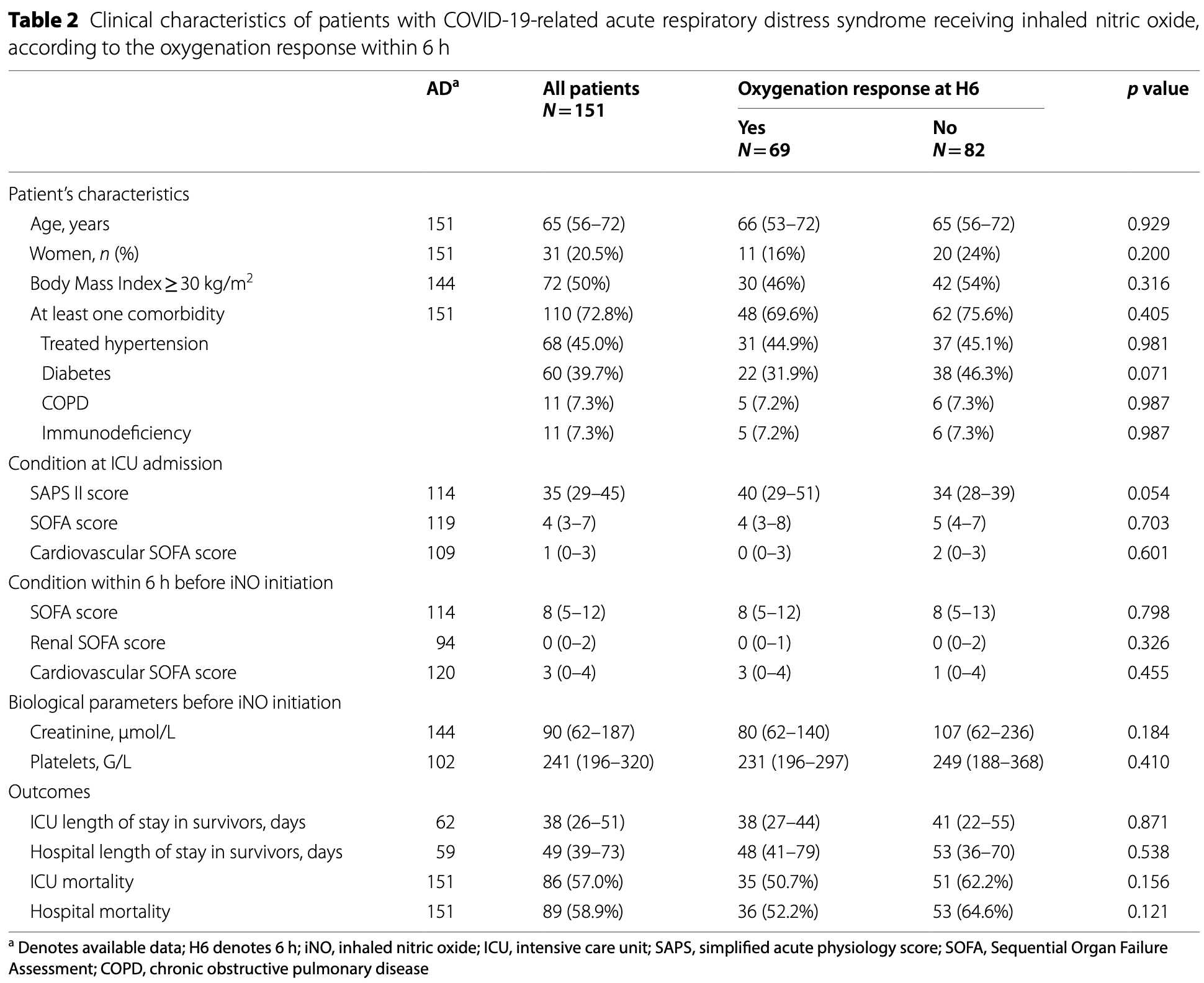
Retrospective 300 COVID-19 ARDS ICU patients showing improved oxygenation (≥20% increase in PaO2/FiO2 ratio) within 6 hours for 46% of patients treated with inhaled nitric oxide (iNO).
Mekontso Dessap et al., 27 Jun 2023, retrospective, France, peer-reviewed, 15 authors, study period 25 February, 2020 - 31 December, 2020.
Contact: armand.dessap@aphp.fr.
Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: treatment modalities, clinical response, and outcomes
Annals of Intensive Care, doi:10.1186/s13613-023-01150-9
Background Inhaled nitric oxide (iNO) has been widely used in patients with COVID-19-related acute respiratory distress syndrome (C-ARDS), though its physiological effects and outcome are debated in this setting. The objective of this cohort study was to describe the modalities of iNO use, clinical response, and outcomes in a large cohort of C-ARDS patients. Methods Multicentre, retrospective cohort study conducted in France.
Results From end February to December 2020, 300 patients (22.3% female) were included, 84.5% were overweight and 69.0% had at least one comorbidity. At ICU admission, their median (IQR) age, SAPS II, and SOFA score were 66 (57-72) years, 37 (29-48), and 5 (3-8), respectively. Patients were all ventilated according to a protective ventilation strategy, and 68% were prone positioned before iNO initiation. At iNO initiation, 2%, 37%, and 61% of patients had mild, moderate, and severe ARDS, respectively. The median duration of iNO treatment was 2.8 (1.1-5.5) days with a median dosage of 10 (7-13) ppm at initiation. Responders (PaO 2 /FiO 2 ratio improving by 20% or more) represented 45.7% of patients at 6 h from iNO initiation. The severity of ARDS was the only predictive factor associated with iNO response. Among all evaluable patients, the crude mortality was not significantly different between responders at 6 h and their counterparts. Of the 62 patients with refractory ARDS (who fulfilled extracorporeal membrane oxygenation criteria before iNO initiation), 32 (51.6%) no longer fulfilled these criteria after 6 h of iNO. The latter showed significantly lower mortality than the other half (who remained ECMO eligible), including after confounder adjustment (adjusted OR: 0.23, 95% CI 0.06, 0.89, p = 0.03).
Conclusions Our study reports the benefits of iNO in improving arterial oxygenation in C-ARDS patients. This improvement seems more relevant in the most severe cases. In patients with ECMO criteria, an iNO-driven improvement in gas exchange was associated with better survival. These results must be confirmed in well-designed prospective studies.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s13613-023-01150-9 . Additional file 1: Table S1 . Clinical characteristics of patients with COVID-19-related acute respiratory distress syndrome receiving inhaled nitric oxide in patients evaluable at 6 h following iNO and the others. Table S2 . Respiratory parameters and modalities of inhaled nitric oxide administration in patients with COVID-19-related acute respiratory distress syndrome in patients evaluable at 6 h following iNO and the others. Table S3 . Oxygenation response to inhaled nitric oxide therapy in patients with COVID-19-related acute respiratory distress syndrome. Table S4 . Change in arterial blood gas variables within 6 h of inhaled nitric oxide therapy in patients with COVID-19-related mild, moderate and severe acute respiratory distress syndrome. Author contributions AMD, LP, MSc, AM, PH, LL and AVB had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. AMD, LP, SN, BM, LH, JFT, JLT, KK, MG, MSl, AM and AVB collected the data. Statistical analyses have been performed under the supervision of MSc according to a predefined statistical plan reviewed by the scientific committee. This scientific committee was composed of LP, AM, AVB and AMD. Interpretation of results, manuscript drafting and submission was the responsibility of the scientific committee...
References
Abou-Arab, Huette, Debouvries, Dupont, Jounieaux et al., Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study, Crit Care
Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary Vascular endothelialitis, thrombosis, and angiogenesis in covid-19, N Engl J Med
Alhazzani, Møller, Arabi, Loeb, Gong, Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive Care Med
Alqahtani, Aldhahir, Ghamdi, Albahrani, Aldraiwiesh et al., Inhaled nitric oxide for clinical management of COVID-19: a systematic review and meta-analysis, Int J Environ Res Public Health
Archer, Sharp, Weir, Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications, Circulation
Bellani, Laffey, Pham, Brochard, Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries, JAMA
Blanch, Joseph, Fernández, Mas, Martinez et al., Hemodynamic and gas exchange responses to inhalation of nitric oxide in patients with the acute respiratory distress syndrome and in hypoxemic patients with chronic obstructive pulmonary disease, Intensive Care Med
Bonizzoli, Lazzeri, Cianchi, Guetti, Fulceri et al., Effects of rescue inhaled nitric oxide on right ventricle and pulmonary circulation in severe COVID-related acute respiratory distress syndrome, J Crit Care
Cavaleiro, Masi, Bagate, Humières, Mekontso, Acute cor pulmonale in Covid-19 related acute respiratory distress syndrome, Crit Care
Combes, Bacry, Fontbonne, Health Data Hub in France, use cases in oncology and radiation oncology, Cancer Radiother J Soc Francaise Radiother Oncol
Combes, Hajage, Capellier, Demoule, Lavoué et al., Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome, N Engl J Med
Dessap, None, Annals of Intensive Care
Feng, Yang, Wen, Liu, Liu et al., Implication of inhaled nitric oxide for the treatment of critically ill COVID-19 patients with pulmonary hypertension, ESC Heart Fail
Ferrari, Santini, Protti, Andreis, Iapichino et al., Inhaled nitric oxide in mechanically ventilated patients with COVID-19, J Crit Care
Gall, Lemeshow, Saulnier, A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study, JAMA
Garfield, Mcfadyen, Briar, Bleakley, Vlachou et al., Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia, Br J Anaesth
Gebistorf, Karam, Wetterslev, Afshari, Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults, Cochrane Database Syst Rev
Group, on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study, Intensive Care Med
Huang, Vignon, Mekontso-Dessap, Tran, Prat et al., Echocardiography findings in COVID-19 patients admitted to intensive care units: a multi-national observational study (the ECHO-COVID study), Intensive Care Med
Keyaerts, Vijgen, Chen, Maes, Hedenstierna et al., Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound, Int J Infect Dis IJID Off Publ Int Soc Infect Dis
Kobayashi, Murata, Nitric oxide inhalation as an interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome, Ann Intensive Care
Lederer, Bell, Branson, Chalmers, Marshall et al., Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals, Ann Am Thorac Soc
Longobardo, Montanari, Shulman, Benhalim, Singer et al., Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome, Br J Anaesth
Lyhne, Kline, Nielsen-Kudsk, Andersen, Pulmonary vasodilation in acute pulmonary embolism-a systematic review, Pulm Circ
Masi, Bagate, Humières, Al-Assaad, Chakra et al., Is hypoxemia explained by intracardiac or intrapulmonary shunt in COVID-19-related acute respiratory distress syndrome?, Ann Intensive Care
Matthay, Aldrich, Gotts, Treatment for severe acute respiratory distress syndrome from COVID-19, Lancet Respir Med
Papazian, Aubron, Brochard, Chiche, Combes et al., Formal guidelines: management of acute respiratory distress syndrome, Ann Intensive Care
Patel, Arachchillage, Ridge, Bianchi, Doyle et al., pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations, Am J Respir Crit Care Med
Peduzzi, Concato, Kemper, Holford, Feinstein, A simulation study of the number of events per variable in logistic regression analysis, J Clin Epidemiol
Petit, Mekontso-Dessap, Masi, Legras, Vignon et al., Evaluation of right ventricular function and driving pressure with blood gas analysis could better select patients eligible for VV ECMO in severe ARDS, Crit Care Lond Engl
Poonam, Koscik, Nguyen, Rikhi, Lin, Nitric oxide versus epoprostenol for refractory hypoxemia in Covid-19, PLoS ONE
Ranieri, Rubenfeld, Thompson, Ferguson, Acute respiratory distress syndrome: the Berlin Definition, JAMA
Robba, Ball, Battaglini, Cardim, Moncalvo et al., Early effects of ventilatory rescue therapies on systemic and cerebral oxygenation in mechanically ventilated COVID-19 patients with acute respiratory distress syndrome: a prospective observational study, Crit Care Lond Engl
Tavazzi, Pozzi, Mongodi, Dammassa, Romito et al., Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia, Crit Care
Vincent, Moreno, Takala, Willatts, Mendonça et al., The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, Intensive Care Med
Vittinghoff, Mcculloch, Relaxing the rule of ten events per variable in logistic and Cox regression, Am J Epidemiol
Åkerström, Mousavi-Jazi, Klingström, Leijon, Lundkvist et al., Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus, J Virol
DOI record:
{
"DOI": "10.1186/s13613-023-01150-9",
"ISSN": [
"2110-5820"
],
"URL": "http://dx.doi.org/10.1186/s13613-023-01150-9",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Inhaled nitric oxide (iNO) has been widely used in patients with COVID-19-related acute respiratory distress syndrome (C-ARDS), though its physiological effects and outcome are debated in this setting. The objective of this cohort study was to describe the modalities of iNO use, clinical response, and outcomes in a large cohort of C-ARDS patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Multicentre, retrospective cohort study conducted in France.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>From end February to December 2020, 300 patients (22.3% female) were included, 84.5% were overweight and 69.0% had at least one comorbidity. At ICU admission, their median (IQR) age, SAPS II, and SOFA score were 66 (57–72) years, 37 (29–48), and 5 (3–8), respectively. Patients were all ventilated according to a protective ventilation strategy, and 68% were prone positioned before iNO initiation. At iNO initiation, 2%, 37%, and 61% of patients had mild, moderate, and severe ARDS, respectively. The median duration of iNO treatment was 2.8 (1.1–5.5) days with a median dosage of 10 (7–13) ppm at initiation. Responders (PaO<jats:sub>2</jats:sub>/FiO<jats:sub>2</jats:sub> ratio improving by 20% or more) represented 45.7% of patients at 6 h from iNO initiation. The severity of ARDS was the only predictive factor associated with iNO response. Among all evaluable patients, the crude mortality was not significantly different between responders at 6 h and their counterparts. Of the 62 patients with refractory ARDS (who fulfilled extracorporeal membrane oxygenation criteria before iNO initiation), 32 (51.6%) no longer fulfilled these criteria after 6 h of iNO. The latter showed significantly lower mortality than the other half (who remained ECMO eligible), including after confounder adjustment (adjusted OR: 0.23, 95% CI 0.06, 0.89, <jats:italic>p</jats:italic> = 0.03).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Our study reports the benefits of iNO in improving arterial oxygenation in C-ARDS patients. This improvement seems more relevant in the most severe cases. In patients with ECMO criteria, an iNO-driven improvement in gas exchange was associated with better survival. These results must be confirmed in well-designed prospective studies.</jats:p>\n </jats:sec>",
"alternative-id": [
"1150"
],
"article-number": "57",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "4 February 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "7 June 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "27 June 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This retrospective cohort study was submitted to Health Data Hub, the regulatory body in charge of validating projects carried out on existing databases in France, in April 2021. Included patients did not object to the use of their personal health data."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "AMD, AVB, LP and AM received consulting fees from Air Liquide Healthcare, which funded the study. MSc, LL and PH are employed by Air Liquide Healthcare."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-5961-5577",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mekontso Dessap",
"given": "Armand",
"sequence": "first"
},
{
"affiliation": [],
"family": "Papazian",
"given": "Laurent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaller",
"given": "Manuella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nseir",
"given": "Saad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Megarbane",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haudebourg",
"given": "Luc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Timsit",
"given": "Jean-François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teboul",
"given": "Jean-Louis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuteifan",
"given": "Khaldoun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gainnier",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slama",
"given": "Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Houeto",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lecourt",
"given": "Laurent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mercat",
"given": "Alain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vieillard-Baron",
"given": "Antoine",
"sequence": "additional"
}
],
"container-title": "Annals of Intensive Care",
"container-title-short": "Ann. Intensive Care",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T10:02:15Z",
"timestamp": 1687860135000
},
"deposited": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T10:09:45Z",
"timestamp": 1687860585000
},
"funder": [
{
"name": "Air Liquide Healthcare"
}
],
"indexed": {
"date-parts": [
[
2025,
10,
18
]
],
"date-time": "2025-10-18T21:03:19Z",
"timestamp": 1760821399070,
"version": "3.37.3"
},
"is-referenced-by-count": 19,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
6,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T00:00:00Z",
"timestamp": 1687824000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T00:00:00Z",
"timestamp": 1687824000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13613-023-01150-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13613-023-01150-9/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13613-023-01150-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2023,
6,
27
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
27
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1002/14651858.CD002787.pub3",
"doi-asserted-by": "crossref",
"key": "1150_CR1",
"unstructured": "Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;6:CD002787."
},
{
"DOI": "10.1186/s13613-019-0540-9",
"author": "L Papazian",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "Ann Intensive Care",
"key": "1150_CR2",
"unstructured": "Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1007/s00134-020-06022-5",
"author": "W Alhazzani",
"doi-asserted-by": "publisher",
"first-page": "854",
"journal-title": "Intensive Care Med",
"key": "1150_CR3",
"unstructured": "Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–87.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30127-2",
"author": "MA Matthay",
"doi-asserted-by": "publisher",
"first-page": "433",
"journal-title": "Lancet Respir Med",
"key": "1150_CR4",
"unstructured": "Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8:433–4.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1002/ehf2.13023",
"author": "W-X Feng",
"doi-asserted-by": "publisher",
"first-page": "714",
"journal-title": "ESC Heart Fail",
"key": "1150_CR5",
"unstructured": "Feng W-X, Yang Y, Wen J, Liu Y-X, Liu L, Feng C. Implication of inhaled nitric oxide for the treatment of critically ill COVID-19 patients with pulmonary hypertension. ESC Heart Fail. 2021;8:714–8.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1186/s13613-020-00681-9",
"author": "J Kobayashi",
"doi-asserted-by": "publisher",
"first-page": "61",
"journal-title": "Ann Intensive Care",
"key": "1150_CR6",
"unstructured": "Kobayashi J, Murata I. Nitric oxide inhalation as an interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome. Ann Intensive Care. 2020;10:61.",
"volume": "10",
"year": "2020"
},
{
"author": "E Keyaerts",
"first-page": "223",
"journal-title": "Int J Infect Dis IJID Off Publ Int Soc Infect Dis",
"key": "1150_CR7",
"unstructured": "Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2004;8:223–6.",
"volume": "8",
"year": "2004"
},
{
"DOI": "10.1128/JVI.79.3.1966-1969.2005",
"author": "S Åkerström",
"doi-asserted-by": "publisher",
"first-page": "1966",
"journal-title": "J Virol",
"key": "1150_CR8",
"unstructured": "Åkerström S, Mousavi-Jazi M, Klingström J, Leijon M, Lundkvist Å, Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol. 2005;79:1966–9.",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.1016/j.bja.2020.11.006",
"author": "B Garfield",
"doi-asserted-by": "publisher",
"first-page": "e72",
"journal-title": "Br J Anaesth",
"key": "1150_CR9",
"unstructured": "Garfield B, McFadyen C, Briar C, Bleakley C, Vlachou A, Baldwin M, et al. Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia. Br J Anaesth. 2021;126:e72–5.",
"volume": "126",
"year": "2021"
},
{
"DOI": "10.1016/j.bja.2020.10.011",
"author": "A Longobardo",
"doi-asserted-by": "publisher",
"first-page": "e44",
"journal-title": "Br J Anaesth",
"key": "1150_CR10",
"unstructured": "Longobardo A, Montanari C, Shulman R, Benhalim S, Singer M, Arulkumaran N. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2021;126:e44–6.",
"volume": "126",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03371-x",
"author": "O Abou-Arab",
"doi-asserted-by": "publisher",
"first-page": "645",
"journal-title": "Crit Care",
"key": "1150_CR11",
"unstructured": "Abou-Arab O, Huette P, Debouvries F, Dupont H, Jounieaux V, Mahjoub Y. Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study. Crit Care. 2020;24:645.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1186/s13054-021-03537-1",
"author": "C Robba",
"doi-asserted-by": "publisher",
"first-page": "111",
"journal-title": "Crit Care Lond Engl",
"key": "1150_CR12",
"unstructured": "Robba C, Ball L, Battaglini D, Cardim D, Moncalvo E, Brunetti I, et al. Early effects of ventilatory rescue therapies on systemic and cerebral oxygenation in mechanically ventilated COVID-19 patients with acute respiratory distress syndrome: a prospective observational study. Crit Care Lond Engl. 2021;25:111.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1016/j.jcrc.2020.08.007",
"author": "M Ferrari",
"doi-asserted-by": "publisher",
"first-page": "159",
"journal-title": "J Crit Care",
"key": "1150_CR13",
"unstructured": "Ferrari M, Santini A, Protti A, Andreis DT, Iapichino G, Castellani G, et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care. 2020;60:159–60.",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.3390/ijerph191912803",
"author": "JS Alqahtani",
"doi-asserted-by": "publisher",
"first-page": "12803",
"journal-title": "Int J Environ Res Public Health",
"key": "1150_CR14",
"unstructured": "Alqahtani JS, Aldhahir AM, Al Ghamdi SS, AlBahrani S, AlDraiwiesh IA, Alqarni AA, et al. Inhaled nitric oxide for clinical management of COVID-19: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:12803.",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.047915",
"author": "SL Archer",
"doi-asserted-by": "publisher",
"first-page": "101",
"journal-title": "Circulation",
"key": "1150_CR15",
"unstructured": "Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications. Circulation. 2020;142:101–4.",
"volume": "142",
"year": "2020"
},
{
"author": "V Ranieri",
"first-page": "2526",
"journal-title": "JAMA",
"key": "1150_CR16",
"unstructured": "Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.",
"volume": "307",
"year": "2012"
},
{
"author": "S Combes",
"first-page": "762",
"journal-title": "Cancer Radiother J Soc Francaise Radiother Oncol",
"key": "1150_CR17",
"unstructured": "Combes S, Bacry E, Fontbonne C. Health Data Hub in France, use cases in oncology and radiation oncology. Cancer Radiother J Soc Francaise Radiother Oncol. 2020;24:762–7.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1001/jama.1993.03510240069035",
"author": "JR Le Gall",
"doi-asserted-by": "publisher",
"first-page": "2957",
"journal-title": "JAMA",
"key": "1150_CR18",
"unstructured": "Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.",
"volume": "270",
"year": "1993"
},
{
"DOI": "10.1007/BF01709751",
"author": "JL Vincent",
"doi-asserted-by": "publisher",
"first-page": "707",
"journal-title": "Intensive Care Med",
"key": "1150_CR19",
"unstructured": "Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.",
"volume": "22",
"year": "1996"
},
{
"key": "1150_CR20",
"unstructured": "Recommandations d’experts portant sur la prise en charge en réanimation des patients infectés à SARS-CoV2. SRLF-SFAR-GFRUP-SPILF-SPLF. Mise en oeuvre avec la mission COREB nationale. 2020."
},
{
"DOI": "10.1007/s001340050290",
"author": "L Blanch",
"doi-asserted-by": "publisher",
"first-page": "51",
"journal-title": "Intensive Care Med",
"key": "1150_CR21",
"unstructured": "Blanch L, Joseph D, Fernández R, Mas A, Martinez M, Vallés J, et al. Hemodynamic and gas exchange responses to inhalation of nitric oxide in patients with the acute respiratory distress syndrome and in hypoxemic patients with chronic obstructive pulmonary disease. Intensive Care Med. 1997;23:51–7.",
"volume": "23",
"year": "1997"
},
{
"DOI": "10.1056/NEJMoa1800385",
"author": "A Combes",
"doi-asserted-by": "publisher",
"first-page": "1965",
"journal-title": "N Engl J Med",
"key": "1150_CR22",
"unstructured": "Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75.",
"volume": "378",
"year": "2018"
},
{
"DOI": "10.1186/s13054-020-03222-9",
"author": "G Tavazzi",
"doi-asserted-by": "publisher",
"first-page": "508",
"journal-title": "Crit Care",
"key": "1150_CR23",
"unstructured": "Tavazzi G, Pozzi M, Mongodi S, Dammassa V, Romito G, Mojoli F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care. 2020;24:508.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1513/AnnalsATS.201808-564PS",
"author": "DJ Lederer",
"doi-asserted-by": "publisher",
"first-page": "22",
"journal-title": "Ann Am Thorac Soc",
"key": "1150_CR24",
"unstructured": "Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16:22–8.",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.1007/s00134-020-06294-x",
"doi-asserted-by": "crossref",
"key": "1150_CR25",
"unstructured": "COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2020;47:60–73."
},
{
"DOI": "10.1001/jama.2016.0291",
"author": "G Bellani",
"doi-asserted-by": "publisher",
"first-page": "788",
"journal-title": "JAMA",
"key": "1150_CR26",
"unstructured": "Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1056/NEJMoa2015432",
"author": "M Ackermann",
"doi-asserted-by": "publisher",
"first-page": "120",
"issue": "2",
"journal-title": "N Engl J Med",
"key": "1150_CR27",
"unstructured": "Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120–8.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202004-1412OC",
"author": "BV Patel",
"doi-asserted-by": "publisher",
"first-page": "690",
"journal-title": "Am J Respir Crit Care Med",
"key": "1150_CR28",
"unstructured": "Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, et al. pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–9.",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1186/s13613-020-00726-z",
"author": "P Masi",
"doi-asserted-by": "publisher",
"first-page": "108",
"journal-title": "Ann Intensive Care",
"key": "1150_CR29",
"unstructured": "Masi P, Bagate F, d’Humières T, Al-Assaad L, Abou Chakra L, Derumeaux G, et al. Is hypoxemia explained by intracardiac or intrapulmonary shunt in COVID-19-related acute respiratory distress syndrome? Ann Intensive Care. 2020;10:108.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1007/s00134-022-06685-2",
"doi-asserted-by": "crossref",
"key": "1150_CR30",
"unstructured": "Huang S, Vignon P, Mekontso-Dessap A, Tran S, Prat G, Chew M, et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: a multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022;48:667–78."
},
{
"DOI": "10.1186/s13054-021-03756-6",
"author": "P Cavaleiro",
"doi-asserted-by": "publisher",
"first-page": "346",
"journal-title": "Crit Care",
"key": "1150_CR31",
"unstructured": "Cavaleiro P, Masi P, Bagate F, d’Humières T, Mekontso DA. Acute cor pulmonale in Covid-19 related acute respiratory distress syndrome. Crit Care. 2021;25:346.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1016/j.jcrc.2022.153987",
"author": "M Bonizzoli",
"doi-asserted-by": "publisher",
"first-page": "153987",
"journal-title": "J Crit Care",
"key": "1150_CR32",
"unstructured": "Bonizzoli M, Lazzeri C, Cianchi G, Guetti C, Fulceri GE, Socci F, et al. Effects of rescue inhaled nitric oxide on right ventricle and pulmonary circulation in severe COVID-related acute respiratory distress syndrome. J Crit Care. 2022;72:153987.",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1177/2045894019899775",
"author": "MD Lyhne",
"doi-asserted-by": "publisher",
"first-page": "204589401989977",
"journal-title": "Pulm Circ",
"key": "1150_CR33",
"unstructured": "Lyhne MD, Kline JA, Nielsen-Kudsk JE, Andersen A. Pulmonary vasodilation in acute pulmonary embolism—a systematic review. Pulm Circ. 2020;10:2045894019899775.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1186/s13054-021-03646-x",
"author": "M Petit",
"doi-asserted-by": "publisher",
"first-page": "220",
"journal-title": "Crit Care Lond Engl",
"key": "1150_CR34",
"unstructured": "Petit M, Mekontso-Dessap A, Masi P, Legras A, Vignon P, Vieillard-Baron A. Evaluation of right ventricular function and driving pressure with blood gas analysis could better select patients eligible for VV ECMO in severe ARDS. Crit Care Lond Engl. 2021;25:220.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0270646",
"author": "PBH Poonam",
"doi-asserted-by": "publisher",
"first-page": "e0270646",
"journal-title": "PLoS ONE",
"key": "1150_CR35",
"unstructured": "Poonam PBH, Koscik R, Nguyen T, Rikhi S, Lin H-M. Nitric oxide versus epoprostenol for refractory hypoxemia in Covid-19. PLoS ONE. 2022;17:e0270646.",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/S0895-4356(96)00236-3",
"author": "P Peduzzi",
"doi-asserted-by": "publisher",
"first-page": "1373",
"journal-title": "J Clin Epidemiol",
"key": "1150_CR36",
"unstructured": "Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9.",
"volume": "49",
"year": "1996"
},
{
"DOI": "10.1093/aje/kwk052",
"author": "E Vittinghoff",
"doi-asserted-by": "publisher",
"first-page": "710",
"journal-title": "Am J Epidemiol",
"key": "1150_CR37",
"unstructured": "Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–8.",
"volume": "165",
"year": "2007"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-023-01150-9"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: treatment modalities, clinical response, and outcomes",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}