Sofosbuvir/Ledipasvir in Combination or Nitazoxanide Alone are Safe and Efficient Treatments for COVID-19 Infection: A Randomized Controlled Trial for Repurposing antivirals
et al., Arab Journal of Gastroenterology, doi:10.1016/j.ajg.2022.04.005, NCT04498936, May 2022
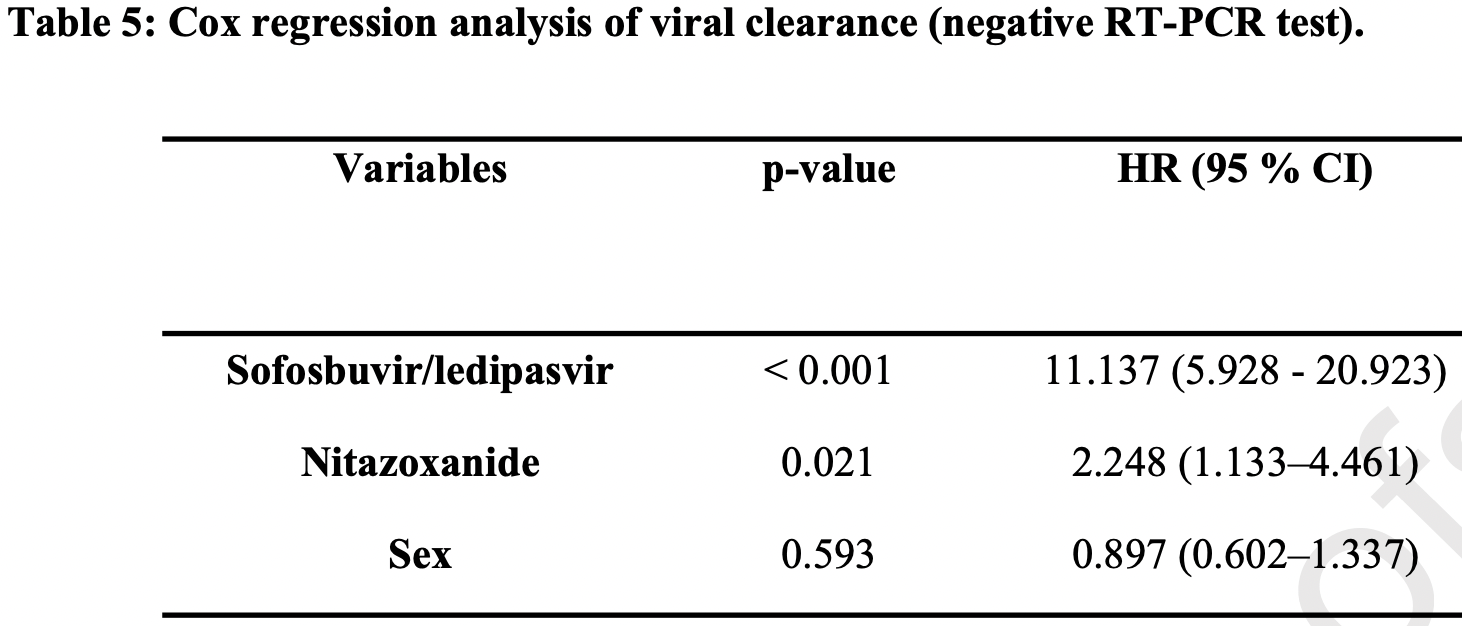
RCT with 77 nitazoxanide, 70 sofosbuvir/ledipasvir, and 73 SOC patients in Egypt, showing faster viral clearance with nitazoxanide and with sofosbuvir/ledipasvir. There was no mortality or progression to severe COVID-19 or ICU admission. Nitazoxanide 500mg qid for 14 days. SOC included vitamin C and zinc.
|
risk of no viral clearance, 55.5% lower, HR 0.44, p = 0.02, treatment 77, control 73, inverted to make HR<1 favor treatment, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Medhat et al., 6 May 2022, Randomized Controlled Trial, Egypt, peer-reviewed, 20 authors, study period July 2020 - October 2021, trial NCT04498936 (history).
Sofosbuvir/ledipasvir in combination or nitazoxanide alone are safe and efficient treatments for COVID-19 infection: A randomized controlled trial for repurposing antivirals
Arab Journal of Gastroenterology, doi:10.1016/j.ajg.2022.04.005
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Aghemo, Francesco, New horizons in hepatitis C antiviral therapy with direct-acting antivirals, Hepatology
Agostini, Pruijssers, Chappell, Gribble, Lu et al., Small-molecule antiviral β-d-N 4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance, Journal of virology
Ahmad, Dwivedy, Mariadasse, Tiwari, Kar et al., Prediction of Small Molecule Inhibitors Targeting the Severe Acute Respiratory Syndrome Coronavirus-2 RNA-dependent RNA Polymerase, ACS omega
Ashour, Elkhatib, Rahman, Elshabrawy, Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks, Pathogens
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-preliminary report, The New England journal of medicine
Bernard, Artigas, Brigham, Carlet, Falke et al., The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination, American journal of respiratory and critical care medicine
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, New England Journal of Medicine
Chen, Yiu, Wong, Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates
Crotty, Cameron, Andino, RNA virus error catastrophe: direct molecular test by using ribavirin, Proceedings of the National Academy of Sciences
El-Bendary, Abd-Elsalam, Elbaz, El-Akel, Elhadidy, Efficacy of combined Sofosbuvir and Daclatasvir in the treatment of COVID-19 patients with pneumonia: a multicenter Egyptian study, Expert Review of Anti-infective Therapy
Elalfy, Besheer, El-Mesery, El-Gilany, Soliman et al., Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19, Journal of medical virology
Elfiky, Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study, Life Sci
Eslami, Mousaviasl, Radmanesh, Jelvay, Bitaraf et al., The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19, Journal of Antimicrobial Chemotherapy
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proceedings of the Japan Academy, Series B
Haffizulla, Hartman, Hoppers, Resnick, Samudrala et al., A randomized, double-blind, placebo controlled clinical trial of nitazoxanide in adults and adolescents with acute uncomplicated influenza, Lancet Infect Dis
Hosseini, Malektojari, Ghazizadeh, Hassaniazad, Davoodian et al., The efficacy and safety of Ivermectin in patients with mild and moderate COVID-19: A structured summary of a study protocol for a randomized controlled trial, Trials
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clinical Infectious Diseases
Jockusch, Tao, Li, Chien, Kumar et al., Sofosbuvir terminated RNA is more resistant to SARS-CoV-2 proofreader than RNA terminated by Remdesivir, Scientific reports
Ju, Kumar, Li, Jockusch, Russo, Nucleotide Analogues as Inhibitors of Viral Polymerases, bioRxiv
Kirchdoerfer, Ward, Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors, Nature communications
Lam, Zheng, Forestieri, Balgi, Nodwell et al., Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis, PLoS pathogens
Li, Xie, Lin, Cai, Wen et al., Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial, Med
Nourian, Khalili, Ahmadinejad, Kouchak, Jafari et al., Efficacy and safety of sofosbuvir/ledipasvir in treatment of patients with COVID-19
Omran, Alboraie, Zayed, Wifi, Naguib et al., Towards hepatitis C virus elimination: Egyptian experience, achievements and limitations, World journal of gastroenterology
Organization, Therapeutics and COVID-19: living guideline
Organization, Therapeutics and COVID-19: living guideline
Paules, Hd, Fauci, Coronavirus infections-more than just the common cold, Jama
Pfizer, Pfizer's novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study
Rajoli, Pertinez, Arshad, Box, Tatham et al., Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, medRxiv
Rocco, Silva, Cruz, Junior, Tierno et al., Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial, European Respiratory Journal
Roozbeh, Saeedi, Alizadeh-Navaei, Hedayatizadeh-Omran, Merat et al., Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial, Journal of Antimicrobial Chemotherapy
Rossignol, Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus, Journal of infection and public health
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral research
Sadeghi, Asgari, Norouzi, Kheiri, Anushirvani et al., Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial, Journal of Antimicrobial Chemotherapy
Sheahan, Sims, Zhou, Graham, Pruijssers et al., An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Science translational medicine
Shi, Wang, Cai, -P, Deng et al., An overview of COVID-19, Journal of Zhejiang University Science B
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virology Journal
Singer, Deutschman, Seymour, Shankar-Hari, Annane et al., The third international consensus definitions for sepsis and septic shock (Sepsis-3), Jama
Singh, Singh, Singh, Misra, Molnupiravir in COVID-19: a systematic review of literature, Diabetes & Metabolic Syndrome
Wahl, Gralinski, Johnson, Yao, Kovarova et al., SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, The Lancet
Warren, Jordan, Lo, Ray, Mackman et al., Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys, Nature
Who, -ncov/solidarity-clinical-trial-for-covid-19-treatments
Wu, Liu, Yang, Zhang, Zhong et al., Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharmaceutica Sinica B
DOI record:
{
"DOI": "10.1016/j.ajg.2022.04.005",
"ISSN": [
"1687-1979"
],
"URL": "http://dx.doi.org/10.1016/j.ajg.2022.04.005",
"alternative-id": [
"S1687197922000326"
],
"author": [
{
"affiliation": [],
"family": "Medhat",
"given": "Mohamed A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "El-Kassas",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karam-Allah",
"given": "Haidi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Shafie",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abd-Elsalam",
"given": "Sherief",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moustafa",
"given": "Ehab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassany",
"given": "Sahar M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salama",
"given": "Marwa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abd Elghafar",
"given": "Mohamed S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sayed",
"given": "Hamdy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badr",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamal",
"given": "Dalia T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shamseldeen",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ossimi",
"given": "Ashima'a",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moaz",
"given": "Inas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "El-deen Esmael",
"given": "Hossam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ezz Eldin",
"given": "Azza M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ezzat",
"given": "Sameera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelghaffar",
"given": "Hossam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelghaffar",
"given": "Khaled",
"sequence": "additional"
}
],
"container-title": "Arab Journal of Gastroenterology",
"container-title-short": "Arab Journal of Gastroenterology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
6
]
],
"date-time": "2022-05-06T09:17:16Z",
"timestamp": 1651828636000
},
"deposited": {
"date-parts": [
[
2022,
5,
6
]
],
"date-time": "2022-05-06T09:18:03Z",
"timestamp": 1651828683000
},
"indexed": {
"date-parts": [
[
2022,
5,
6
]
],
"date-time": "2022-05-06T09:42:20Z",
"timestamp": 1651830140646
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
1
]
],
"date-time": "2022-05-01T00:00:00Z",
"timestamp": 1651363200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1687197922000326?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1687197922000326?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.3390/pathogens9030186",
"article-title": "Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks",
"author": "Ashour",
"doi-asserted-by": "crossref",
"first-page": "186",
"issue": "3",
"journal-title": "Pathogens",
"key": "10.1016/j.ajg.2022.04.005_b0005",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1631/jzus.B2000083",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0010",
"unstructured": "Shi Y, Wang G, Cai X-p, Deng J-w, Zheng L, Zhu H-h, et al. An overview of COVID-19. Journal of Zhejiang University Science B 2020:1."
},
{
"key": "10.1016/j.ajg.2022.04.005_b0015",
"unstructured": "WHO. Global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments 2020."
},
{
"DOI": "10.1186/s13063-020-04988-7",
"article-title": "The efficacy and safety of Ivermectin in patients with mild and moderate COVID-19: A structured summary of a study protocol for a randomized controlled trial",
"author": "Hosseini",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Trials",
"key": "10.1016/j.ajg.2022.04.005_b0020",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.0757",
"article-title": "Coronavirus infections—more than just the common cold",
"author": "Paules",
"doi-asserted-by": "crossref",
"first-page": "707",
"issue": "8",
"journal-title": "Jama",
"key": "10.1016/j.ajg.2022.04.005_b0025",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1038/s41467-019-10280-3",
"article-title": "Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors",
"author": "Kirchdoerfer",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Nature communications",
"key": "10.1016/j.ajg.2022.04.005_b0030",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "FURUTA",
"doi-asserted-by": "crossref",
"first-page": "449",
"issue": "7",
"journal-title": "Proceedings of the Japan Academy, Series B",
"key": "10.1016/j.ajg.2022.04.005_b0035",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1038/nature17180",
"article-title": "Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys",
"author": "Warren",
"doi-asserted-by": "crossref",
"first-page": "381",
"issue": "7594",
"journal-title": "Nature",
"key": "10.1016/j.ajg.2022.04.005_b0040",
"volume": "531",
"year": "2016"
},
{
"DOI": "10.1101/2020.01.30.927574",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0045",
"unstructured": "Ju J, Kumar S, Li X, Jockusch S, Russo JJ. Nucleotide Analogues as Inhibitors of Viral Polymerases. bioRxiv 2020:2020.2001.2030.927574."
},
{
"DOI": "10.12688/f1000research.22457.1",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0050",
"unstructured": "Chen Y, Yiu C, Wong K. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates [version 1; peer review: 3 approved]. F1000Research 2020;9."
},
{
"DOI": "10.1016/j.lfs.2020.117592",
"article-title": "Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study",
"author": "Elfiky",
"doi-asserted-by": "crossref",
"first-page": "117592",
"journal-title": "Life Sci",
"key": "10.1016/j.ajg.2022.04.005_b0055",
"volume": "253",
"year": "2020"
},
{
"DOI": "10.1002/hep.26371",
"article-title": "New horizons in hepatitis C antiviral therapy with direct-acting antivirals",
"author": "Aghemo",
"doi-asserted-by": "crossref",
"first-page": "428",
"issue": "1",
"journal-title": "Hepatology",
"key": "10.1016/j.ajg.2022.04.005_b0060",
"volume": "58",
"year": "2013"
},
{
"DOI": "10.3748/wjg.v24.i38.4330",
"article-title": "Towards hepatitis C virus elimination: Egyptian experience, achievements and limitations",
"author": "Omran",
"doi-asserted-by": "crossref",
"first-page": "4330",
"issue": "38",
"journal-title": "World journal of gastroenterology",
"key": "10.1016/j.ajg.2022.04.005_b0065",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.1016/j.apsb.2020.02.008",
"article-title": "Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "766",
"issue": "5",
"journal-title": "Acta Pharmaceutica Sinica B",
"key": "10.1016/j.ajg.2022.04.005_b0070",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.jiph.2016.04.001",
"article-title": "Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "227",
"issue": "3",
"journal-title": "Journal of infection and public health",
"key": "10.1016/j.ajg.2022.04.005_b0075",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"article-title": "A randomized, double-blind, placebo controlled clinical trial of nitazoxanide in adults and adolescents with acute uncomplicated influenza",
"author": "Haffizulla",
"doi-asserted-by": "crossref",
"first-page": "609",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ajg.2022.04.005_b0080",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.22541/au.158938595.50403411",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0085",
"unstructured": "Rajoli RK, Pertinez H, Arshad U, Box H, Tatham L, Curley P, et al. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. medRxiv 2020."
},
{
"key": "10.1016/j.ajg.2022.04.005_b0090",
"unstructured": "Organization WH. Therapeutics and COVID-19: living guideline, 24 September 2021: World Health Organization; 2021."
},
{
"author": "Organization",
"key": "10.1016/j.ajg.2022.04.005_b0095",
"series-title": "Therapeutics and COVID-19: living guideline",
"year": "2021"
},
{
"DOI": "10.1164/ajrccm.149.3.7509706",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0100",
"unstructured": "Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine 1994;149:818-824."
},
{
"DOI": "10.1001/jama.2016.0287",
"article-title": "The third international consensus definitions for sepsis and septic shock (Sepsis-3)",
"author": "Singer",
"doi-asserted-by": "crossref",
"first-page": "801",
"issue": "8",
"journal-title": "Jama",
"key": "10.1016/j.ajg.2022.04.005_b0105",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1021/acsomega.0c02096",
"article-title": "Prediction of Small Molecule Inhibitors Targeting the Severe Acute Respiratory Syndrome Coronavirus-2 RNA-dependent RNA Polymerase",
"author": "Ahmad",
"doi-asserted-by": "crossref",
"first-page": "18356",
"issue": "29",
"journal-title": "ACS omega",
"key": "10.1016/j.ajg.2022.04.005_b0110",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—preliminary report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"journal-title": "The New England journal of medicine",
"key": "10.1016/j.ajg.2022.04.005_b0115",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "The Lancet",
"key": "10.1016/j.ajg.2022.04.005_b0120",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-73641-9",
"article-title": "Sofosbuvir terminated RNA is more resistant to SARS-CoV-2 proofreader than RNA terminated by Remdesivir",
"author": "Jockusch",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Scientific reports",
"key": "10.1016/j.ajg.2022.04.005_b0125",
"volume": "10",
"year": "2020"
},
{
"author": "Nourian",
"first-page": "91",
"key": "10.1016/j.ajg.2022.04.005_b0130",
"series-title": "Efficacy and safety of sofosbuvir/ledipasvir in treatment of patients with COVID-19; A randomized clinical trial",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkaa501",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0135",
"unstructured": "Roozbeh F, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Merat S, Wentzel H, et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. Journal of Antimicrobial Chemotherapy 2020."
},
{
"DOI": "10.1093/jac/dkaa331",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0140",
"unstructured": "Eslami G, Mousaviasl S, Radmanesh E, Jelvay S, Bitaraf S, Simmons B, et al. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. Journal of Antimicrobial Chemotherapy 2020;75:3366-3372."
},
{
"DOI": "10.1093/jac/dkaa334",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0145",
"unstructured": "Sadeghi A, Ali Asgari A, Norouzi A, Kheiri Z, Anushirvani A, Montazeri M, et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. Journal of Antimicrobial Chemotherapy 2020;75:3379-3385."
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"article-title": "Nitazoxanide: a first-in-class broad-spectrum antiviral agent",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "Antiviral research",
"key": "10.1016/j.ajg.2022.04.005_b0150",
"volume": "110",
"year": "2014"
},
{
"DOI": "10.1371/journal.ppat.1002691",
"article-title": "Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis",
"author": "Lam",
"doi-asserted-by": "crossref",
"first-page": "e1002691",
"issue": "5",
"journal-title": "PLoS pathogens",
"key": "10.1016/j.ajg.2022.04.005_b0155",
"volume": "8",
"year": "2012"
},
{
"article-title": "Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial",
"author": "Rocco",
"journal-title": "European Respiratory Journal",
"key": "10.1016/j.ajg.2022.04.005_b0160",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26880",
"article-title": "Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19",
"author": "Elalfy",
"doi-asserted-by": "crossref",
"first-page": "3176",
"issue": "5",
"journal-title": "Journal of medical virology",
"key": "10.1016/j.ajg.2022.04.005_b0165",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.medj.2020.04.001",
"article-title": "Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "105",
"issue": "1",
"journal-title": "Med",
"key": "10.1016/j.ajg.2022.04.005_b0170",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.ajg.2022.04.005_b0175",
"year": "2020"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"article-title": "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Virology Journal",
"key": "10.1016/j.ajg.2022.04.005_b0180",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1176",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0185",
"unstructured": "Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, et al. AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clinical Infectious Diseases 2020."
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"article-title": "Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "1192",
"issue": "10",
"journal-title": "Engineering",
"key": "10.1016/j.ajg.2022.04.005_b0190",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1080/14787210.2021.1950532",
"article-title": "Efficacy of combined Sofosbuvir and Daclatasvir in the treatment of COVID-19 patients with pneumonia: a multicenter Egyptian study",
"author": "El-Bendary",
"doi-asserted-by": "crossref",
"first-page": "291",
"issue": "2",
"journal-title": "Expert Review of Anti-infective Therapy",
"key": "10.1016/j.ajg.2022.04.005_b0195",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1073/pnas.111085598",
"article-title": "RNA virus error catastrophe: direct molecular test by using ribavirin",
"author": "Crotty",
"doi-asserted-by": "crossref",
"first-page": "6895",
"issue": "12",
"journal-title": "Proceedings of the National Academy of Sciences",
"key": "10.1016/j.ajg.2022.04.005_b0200",
"volume": "98",
"year": "2001"
},
{
"DOI": "10.1128/JVI.01348-19",
"article-title": "Small-molecule antiviral β-d-N 4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance",
"author": "Agostini",
"doi-asserted-by": "crossref",
"first-page": "e01348",
"journal-title": "Journal of virology",
"key": "10.1016/j.ajg.2022.04.005_b0205",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"article-title": "SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801",
"author": "Wahl",
"doi-asserted-by": "crossref",
"first-page": "451",
"issue": "7850",
"journal-title": "Nature",
"key": "10.1016/j.ajg.2022.04.005_b0210",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajg.2022.04.005_b0215",
"unstructured": "Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Science translational medicine 2020;12:eabb5883."
},
{
"DOI": "10.1016/j.dsx.2021.102329",
"article-title": "Molnupiravir in COVID-19: a systematic review of literature",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "102329",
"issue": "6",
"journal-title": "Diabetes & Metabolic Syndrome: Clinical Research & Reviews",
"key": "10.1016/j.ajg.2022.04.005_b0220",
"volume": "15",
"year": "2021"
},
{
"key": "10.1016/j.ajg.2022.04.005_b0225",
"unstructured": "Pfizer. Pfizer's novel COVID‐19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC‐HR study."
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1687197922000326"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Gastroenterology"
],
"subtitle": [],
"title": "Sofosbuvir/Ledipasvir in Combination or Nitazoxanide Alone are Safe and Efficient Treatments for COVID-19 Infection: A Randomized Controlled Trial for Repurposing antivirals",
"type": "journal-article"
}