Safety and Tolerability of Multimodal Therapy (Ivermectin, Doxycycline, Vitamin C, Vitamin D, and Zinc) With or Without Famotidine in Australian Patients With COVID-19 Infection: A Pilot Cohort Trial
et al., American Journal of Therapeutics, doi:10.1097/MJT.0000000000002118, PACT, Feb 2026
Famotidine for COVID-19
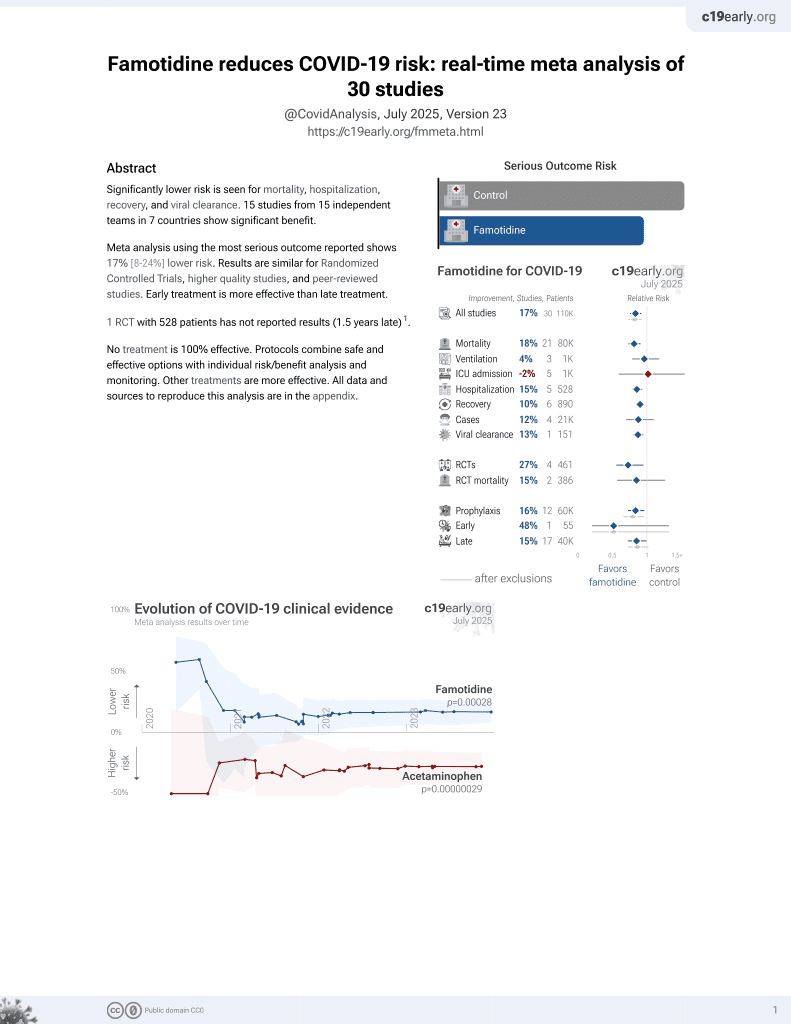
29th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
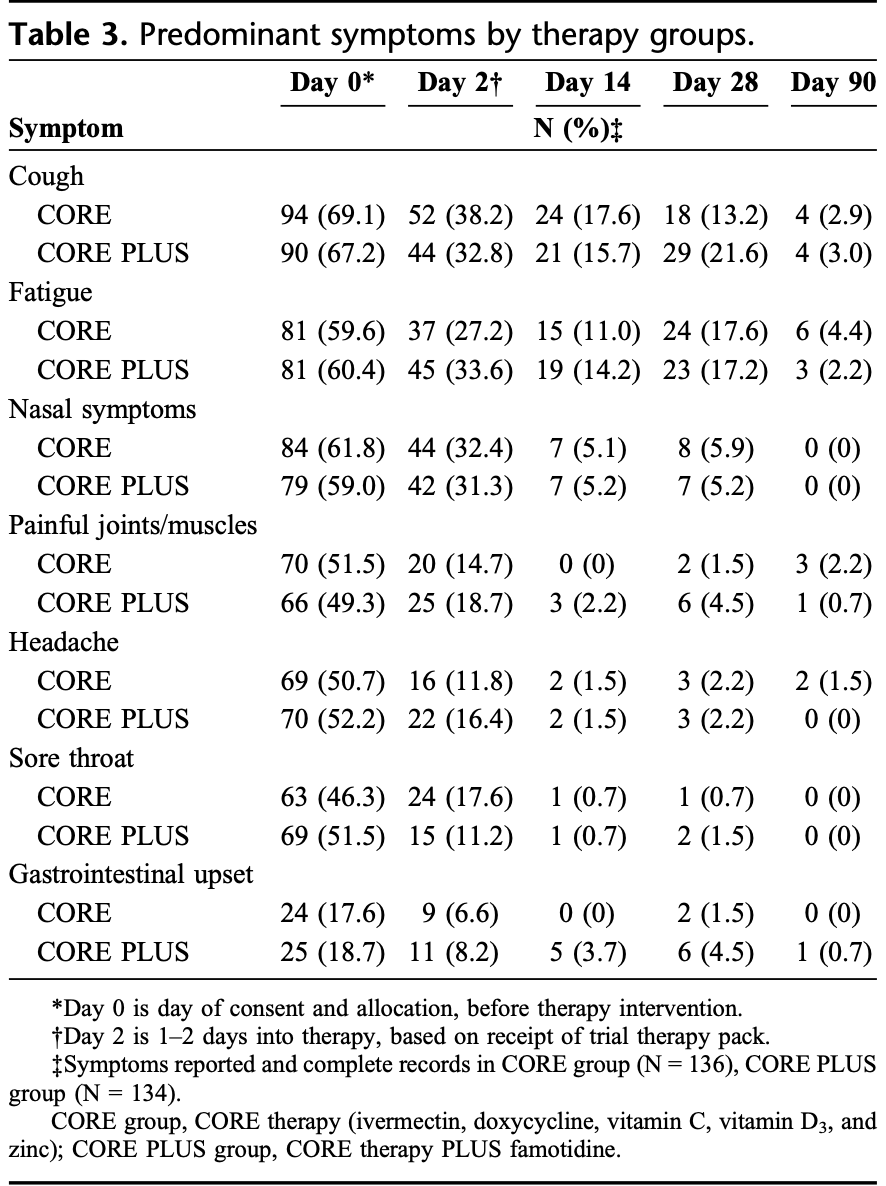
RCT 275 outpatients showing very low hospitalization with combination therapy including ivermectin, doxycycline, vitamin C, vitamin D3, and zinc, with or without famotidine. There was no control group. Only 4 patients were hospitalized within 28 days, all in the famotidine group due to exacerbations of pre-existing conditions. The addition of famotidine improved nasal symptoms and fatigue resolution at day 14 and wellbeing scores at day 90. Most symptoms resolved by day 2 of therapy in both groups. There was no ICU admission, ventilation, or death in either group.
McLindon et al., 16 Feb 2026, Double Blind Randomized Controlled Trial, Australia, peer-reviewed, mean age 59.7, 6 authors, study period April 2022 - February 2023, PACT trial.
Contact: karinried@niim.com.au.
not-yet-known not-yet-known not-yet-known unknown Therapies to prevent progression of COVID-19, including Ivermectin, Doxycycline, Vitamin C, Vitamin D, and Zinc with or without Famotidine: a randomised controlled double-blind multi-centre outpatient cohort study
doi:10.22541/au.176072414.48837718/v1
Background: In 2020, COVID-19 caused by the SARS-CoV-2 emerged as a global pandemic. Combination therapies for viral illnesses may be more effective than single-agent therapies in reducing symptoms and preventing disease progression. Repurposed drugs may offer cost-effective accessible treatment options while other novel therapies are being developed. Study Question: To assess the safety and tolerability of a combination of repurposed drugs and supplements in the early therapy of acute nonhospitalized COVID-19 illness. Study Design: A multicenter pilot cohort study was undertaken. Participants older than 40 years and positive for COVID-19 within 4 days of illness onset received telehealth outpatient care. The multimodal therapy of ivermectin, doxycyline, zinc, vitamin C, and vitamin D 3 was given as core therapy (group-1), or core therapy plus famotidine (group-2) for 10 days in a randomized masked controlled fashion.
Measures and Outcomes: A total of 275 participants, who were either vaccinated (n = 151) or unvaccinated (n = 124) for COVID-19, were randomized into group-1 (n = 137) or group-2 (n = 138). Four participants (1.5%) were hospitalized within the first 28 days.
Results: No viral rebound was experienced by any participants who took the 10-day therapy. Symptoms were reported daily until day 10, and at days 14, 28, and 90. The addition of famotidine resulted in less fatigue and nasal symptoms at day 14. These 5-component and 6-component interventions (ie, core therapy with and without famotidine) were equally safe and well tolerated.
Conclusions: The multimodal therapy for acute COVID-19 was safe and well tolerated. The results justify adequately powered trials of the 5-or 6-component intervention on major early and late outcomes of COVID-19 infections.
Supplementary Table S1a+b , http://links.lww.com/AJT/ A252 ). There were no cases of rebound COVID-19 illness detected in this cohort receiving 10 days of therapy. When analyzing oxygen saturations during the first 14 days of illness, there was no difference (P = 0.312) between treatment groups. Interestingly, oxygen levels were
References
Aggarwal, Molina, Beaty, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, Lancet Infect Dis
Ahmed, A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection, Front Immunol
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Benskin, A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency, Front Public Health
Biber, Harmelin, Lev, The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19-a double-blind, randomized placebo-controlled trial, Int J Infect Dis
Brennan, Nadella, Zhao, Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial, Gut
Butler, Yu, Dorward, Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, openlabel, adaptive platform trial, Lancet Respir Med
Caly, Druce, Catton, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antivir Res
Cerullo, Negro, Parimbelli, The long history of vitamin C: from prevention of the common cold to potential aid in the treatment of COVID-19, Front Immunol
Chang, Nguyen, Martiniuk, Persistence of the omicron variant of SARS-CoV-2 in Australia: the impact of fluctuating social distancing, PLOS Glob Public Health
Chen, Haupert, Zimmermann, Global prevalence of postcoronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review, J Infect Dis
Chiu, Shen, Lo, Effect of famotidine on hospitalized patients with COVID-19: a systematic review and meta-analysis, PLoS One
Davis, Mccorkell, Vogel, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol
Du, Ma, Deng, Comparison of long COVID-19 caused by different SARS-CoV-2 strains: a systematic review and meta-analysis, Int J Environ Res Public Health
Fernandez-De-Las-Penas, Raveendran, Giordano, Long COVID or Post-COVID-19 condition: past, present and future research directions, Microorganisms
Guzzo, Furtek, Porras, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol
Hashim, Maulood, Rasheed, Controlled randomized clinical trial on using Ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq, Iraqi JMS
Hayward, Yu, Little, Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short-and longer-term outcomes, J Infect
Hm, Gareeb, Alqarni, Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives, Front Pharmacol
Lippi, Sanchis-Gomar, Henry, COVID-19 and its long-term sequelae: what do we know in 2023?, Pol Arch Intern Med
Mahmud, Rahman, Alam, Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial, J Int Med Res
Malone, Tisdall, Smith, COVID-19: Famotidine, histamine, mast cells, and mechanisms, Front Pharmacol
Matthew, John, Lynne, Ongoing symptoms and functional impairment 12 weeks after testing positive for SARS-CoV-2 or influenza in Australia: an observational cohort study, BMJ Public Health
Naggie, Boulware, Lindsell, Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial, Jama
Navarro, Camprubi, Requena-Mendez, Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother
Reis, Metzendorf, Kuehn, Nirmatrelvir combined with ritonavir for preventing and treating COVID-19, Cochrane Database Syst Rev
Reis, Silva, Silva, Effect of early treatment with ivermectin among patients with Covid-19, N Engl J Med
Ried, Binjemain, Sali, Therapies to prevent progression of COVID-19, including hydroxychloroquine, azithromycin, zinc, and vitamin D3 with or without intravenous vitamin C: an international, multicenter, randomized trial, Cureus
Shafiee, Athar, Gargari, Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients, Virol J
Sharma, Bhatt, Asdaq, Combined therapy with ivermectin and doxycycline can effectively alleviate the cytokine storm of COVID-19 infection amid vaccination drive: a narrative review, J Infect Public Health
Sobngwi, Zemsi, Guewo, Doxycycline vs hydroxychloro-quine+ azithromycin in the management of COVID-19 patients: an openlabel randomized clinical trial in Sub-Saharan Africa (DOXYCOV), Cureus
Sturgiss, Simpson, Ball, Community-based access to oral antiviral treatments for COVID-19 in Australia, Aust J Gen Pract
Tian, Feng, Chen, Efficacy and safety of molnupiravir treatment for COVID-19: a systematic review and meta-analysis of randomized controlled trials, Int J Antimicrob Agents
Velthuis, Van Den Worm, Sims, Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture, Plos Pathog
DOI record:
{
"DOI": "10.1097/mjt.0000000000002118",
"ISSN": [
"1536-3686"
],
"URL": "http://dx.doi.org/10.1097/MJT.0000000000002118",
"abstract": "<jats:sec>\n <jats:title>Background:</jats:title>\n <jats:p>In 2020, COVID-19 caused by the SARS-CoV-2 emerged as a global pandemic. Combination therapies for viral illnesses may be more effective than single-agent therapies in reducing symptoms and preventing disease progression. Repurposed drugs may offer cost-effective accessible treatment options while other novel therapies are being developed.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Study Question:</jats:title>\n <jats:p>To assess the safety and tolerability of a combination of repurposed drugs and supplements in the early therapy of acute nonhospitalized COVID-19 illness.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Study Design:</jats:title>\n <jats:p>\n A multicenter pilot cohort study was undertaken. Participants older than 40 years and positive for COVID-19 within 4 days of illness onset received telehealth outpatient care. The multimodal therapy of ivermectin, doxycyline, zinc, vitamin C, and vitamin D\n <jats:sub>3</jats:sub>\n was given as core therapy (group-1), or core therapy plus famotidine (group-2) for 10 days in a randomized masked controlled fashion.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Measures and Outcomes:</jats:title>\n <jats:p>A total of 275 participants, who were either vaccinated (n = 151) or unvaccinated (n = 124) for COVID-19, were randomized into group-1 (n = 137) or group-2 (n = 138). Four participants (1.5%) were hospitalized within the first 28 days.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results:</jats:title>\n <jats:p>No viral rebound was experienced by any participants who took the 10-day therapy. Symptoms were reported daily until day 10, and at days 14, 28, and 90. The addition of famotidine resulted in less fatigue and nasal symptoms at day 14. These 5-component and 6-component interventions (ie, core therapy with and without famotidine) were equally safe and well tolerated.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions:</jats:title>\n <jats:p>The multimodal therapy for acute COVID-19 was safe and well tolerated. The results justify adequately powered trials of the 5- or 6-component intervention on major early and late outcomes of COVID-19 infections.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Medical School, University of Queensland, Herston, QLD, Australia;"
}
],
"family": "McLindon",
"given": "Lucas A.",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0003-1054-3490",
"affiliation": [
{
"name": "National Institute of Integrative Medicine (NIIM), Hawthorn, VIC, Australia;"
}
],
"authenticated-orcid": false,
"family": "Ried",
"given": "Karin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bedford Medical Clinic, Clarence Gardens, SA, Australia; and"
}
],
"family": "Wauchope",
"given": "Bruce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute of Integrative Medicine (NIIM), Hawthorn, VIC, Australia;"
}
],
"family": "Murnane",
"given": "Lucia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute of Integrative Medicine (NIIM), Hawthorn, VIC, Australia;"
}
],
"family": "Harradine",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Marion Compounding Pharmacy, Marion, SA, Australia."
}
],
"family": "Seman",
"given": "Josie",
"sequence": "additional"
}
],
"container-title": "American Journal of Therapeutics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"lww.com",
"ovid.com"
]
},
"created": {
"date-parts": [
[
2026,
2,
16
]
],
"date-time": "2026-02-16T05:00:19Z",
"timestamp": 1771218019000
},
"deposited": {
"date-parts": [
[
2026,
2,
16
]
],
"date-time": "2026-02-16T05:00:25Z",
"timestamp": 1771218025000
},
"indexed": {
"date-parts": [
[
2026,
2,
16
]
],
"date-time": "2026-02-16T06:00:23Z",
"timestamp": 1771221623501,
"version": "3.50.1"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
2,
16
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
2,
16
]
],
"date-time": "2026-02-16T00:00:00Z",
"timestamp": 1771200000000
}
}
],
"link": [
{
"URL": "https://journals.lww.com/10.1097/MJT.0000000000002118",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"prefix": "10.1097",
"published": {
"date-parts": [
[
2026,
2,
16
]
]
},
"published-online": {
"date-parts": [
[
2026,
2,
16
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"article-title": "Nirmatrelvir combined with ritonavir for preventing and treating COVID-19",
"author": "Reis",
"first-page": "CD015395",
"journal-title": "Cochrane Database Syst Rev",
"key": "R3-20260216",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.ijantimicag.2023.106870",
"article-title": "Efficacy and safety of molnupiravir treatment for COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Tian",
"doi-asserted-by": "crossref",
"first-page": "106870",
"journal-title": "Int J Antimicrob Agents",
"key": "R4-20260216",
"volume": "62",
"year": "2023"
},
{
"article-title": "Therapies to prevent progression of COVID-19, including hydroxychloroquine, azithromycin, zinc, and vitamin D3 with or without intravenous vitamin C: an international, multicenter, randomized trial",
"author": "Ried",
"first-page": "e19902",
"journal-title": "Cureus",
"key": "R5-20260216",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.574029",
"article-title": "The long history of vitamin C: from prevention of the common cold to potential aid in the treatment of COVID-19",
"author": "Cerullo",
"doi-asserted-by": "crossref",
"first-page": "574029",
"journal-title": "Front Immunol",
"key": "R6-20260216",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.590459",
"article-title": "A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection",
"author": "Ahmed",
"doi-asserted-by": "crossref",
"first-page": "590459",
"journal-title": "Front Immunol",
"key": "R7-20260216",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fpubh.2020.00513",
"article-title": "A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency",
"author": "Benskin",
"doi-asserted-by": "crossref",
"first-page": "513",
"journal-title": "Front Public Health",
"key": "R8-20260216",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1001176",
"article-title": "Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture",
"author": "te Velthuis",
"doi-asserted-by": "crossref",
"first-page": "e1001176",
"journal-title": "Plos Pathog",
"key": "R9-20260216",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antivir Res",
"key": "R10-20260216",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2022.07.003",
"article-title": "The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19–a double-blind, randomized placebo-controlled trial",
"author": "Biber",
"doi-asserted-by": "crossref",
"first-page": "733",
"journal-title": "Int J Infect Dis",
"key": "R11-20260216",
"volume": "122",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"article-title": "A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness",
"author": "Ahmed",
"doi-asserted-by": "crossref",
"first-page": "214",
"journal-title": "Int J Infect Dis",
"key": "R12-20260216",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.22578/IJMS.19.1.14",
"article-title": "Controlled randomized clinical trial on using Ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq",
"author": "Hashim",
"doi-asserted-by": "crossref",
"first-page": "107",
"issue": "1",
"journal-title": "Iraqi JMS",
"key": "R13-20260216",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1186/s12985-022-01829-8",
"article-title": "Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients",
"author": "Shafiee",
"doi-asserted-by": "crossref",
"first-page": "102",
"journal-title": "Virol J",
"key": "R14-20260216",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1177/009127002237994",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo",
"doi-asserted-by": "crossref",
"first-page": "1122",
"journal-title": "J Clin Pharmacol",
"key": "R15-20260216",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1093/jac/dkz524",
"article-title": "Safety of high-dose ivermectin: a systematic review and meta-analysis",
"author": "Navarro",
"doi-asserted-by": "crossref",
"first-page": "827",
"journal-title": "J Antimicrob Chemother",
"key": "R16-20260216",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.642822",
"article-title": "Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives",
"author": "Al-Kuraishy",
"doi-asserted-by": "crossref",
"first-page": "642822",
"journal-title": "Front Pharmacol",
"key": "R17-20260216",
"volume": "12",
"year": "2021"
},
{
"article-title": "Doxycycline vs hydroxychloroquine+ azithromycin in the management of COVID-19 patients: an open-label randomized clinical trial in Sub-Saharan Africa (DOXYCOV)",
"author": "Sobngwi",
"first-page": "e45619",
"journal-title": "Cureus",
"key": "R18-20260216",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.jiph.2022.03.014",
"article-title": "Combined therapy with ivermectin and doxycycline can effectively alleviate the cytokine storm of COVID-19 infection amid vaccination drive: a narrative review",
"author": "Sharma",
"doi-asserted-by": "crossref",
"first-page": "566",
"journal-title": "J Infect Public Health",
"key": "R19-20260216",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1177/03000605211013550",
"article-title": "Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial",
"author": "Mahmud",
"doi-asserted-by": "crossref",
"first-page": "03000605211013550",
"journal-title": "J Int Med Res",
"key": "R20-20260216",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1136/gutjnl-2022-326952",
"article-title": "Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial",
"author": "Brennan",
"doi-asserted-by": "crossref",
"first-page": "879",
"journal-title": "Gut",
"key": "R22-20260216",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0259514",
"article-title": "Effect of famotidine on hospitalized patients with COVID-19: a systematic review and meta-analysis",
"author": "Chiu",
"doi-asserted-by": "crossref",
"first-page": "e0259514",
"journal-title": "PLoS One",
"key": "R23-20260216",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.633680",
"article-title": "COVID-19: Famotidine, histamine, mast cells, and mechanisms",
"author": "Malone",
"doi-asserted-by": "crossref",
"first-page": "633680",
"journal-title": "Front Pharmacol",
"key": "R24-20260216",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiac136",
"article-title": "Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "1593",
"journal-title": "J Infect Dis",
"key": "R25-20260216",
"volume": "226",
"year": "2022"
},
{
"DOI": "10.1136/bmjph-2023-000060",
"article-title": "Ongoing symptoms and functional impairment 12 weeks after testing positive for SARS-CoV-2 or influenza in Australia: an observational cohort study",
"author": "Matthew",
"doi-asserted-by": "crossref",
"first-page": "e000060",
"journal-title": "BMJ Public Health",
"key": "R26-20260216",
"volume": "1",
"year": "2023"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"article-title": "Long COVID: major findings, mechanisms and recommendations",
"author": "Davis",
"doi-asserted-by": "crossref",
"first-page": "133",
"journal-title": "Nat Rev Microbiol",
"key": "R27-20260216",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.31128/AJGP-01-23-6673",
"article-title": "Community-based access to oral antiviral treatments for COVID-19 in Australia",
"author": "Sturgiss",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Aust J Gen Pract",
"key": "R29-20260216",
"volume": "52",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00011-7",
"article-title": "Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "696",
"journal-title": "Lancet Infect Dis",
"key": "R30-20260216",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1371/journal.pgph.0001427",
"article-title": "Persistence of the omicron variant of SARS-CoV-2 in Australia: the impact of fluctuating social distancing",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "e0001427",
"journal-title": "PLOS Glob Public Health",
"key": "R31-20260216",
"volume": "3",
"year": "2023"
},
{
"article-title": "COVID-19 and its long-term sequelae: what do we know in 2023?",
"author": "Lippi",
"first-page": "16402",
"journal-title": "Pol Arch Intern Med",
"key": "R32-20260216",
"volume": "133",
"year": "2023"
},
{
"DOI": "10.3390/ijerph192316010",
"article-title": "Comparison of long COVID-19 caused by different SARS-CoV-2 strains: a systematic review and meta-analysis",
"author": "Du",
"doi-asserted-by": "crossref",
"first-page": "16010",
"journal-title": "Int J Environ Res Public Health",
"key": "R33-20260216",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.3390/microorganisms11122959",
"article-title": "Long COVID or Post-COVID-19 condition: past, present and future research directions",
"author": "Fernandez-de-Las-Penas",
"doi-asserted-by": "crossref",
"first-page": "2959",
"journal-title": "Microorganisms",
"key": "R34-20260216",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with ivermectin among patients with Covid-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"journal-title": "N Engl J Med",
"key": "R35-20260216",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2024.106130",
"article-title": "Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes",
"author": "Hayward",
"doi-asserted-by": "crossref",
"first-page": "106130",
"journal-title": "J Infect",
"key": "R36-20260216",
"volume": "88",
"year": "2024"
},
{
"DOI": "10.1016/S2213-2600(21)00310-6",
"article-title": "Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "1010",
"journal-title": "Lancet Respir Med",
"key": "R37-20260216",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "888",
"journal-title": "Jama",
"key": "R38-20260216",
"volume": "329",
"year": "2023"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/MJT.0000000000002118"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Safety and Tolerability of Multimodal Therapy (Ivermectin, Doxycycline, Vitamin C, Vitamin D, and Zinc) With or Without Famotidine in Australian Patients With COVID-19 Infection: A Pilot Cohort Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1097/lww.0000000000001000"
}
mclindon