The choice of viral load endpoint in early phase trials of COVID-19 treatments aiming to reduce 28-day hospitalization and/or death
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf282, Jun 2025
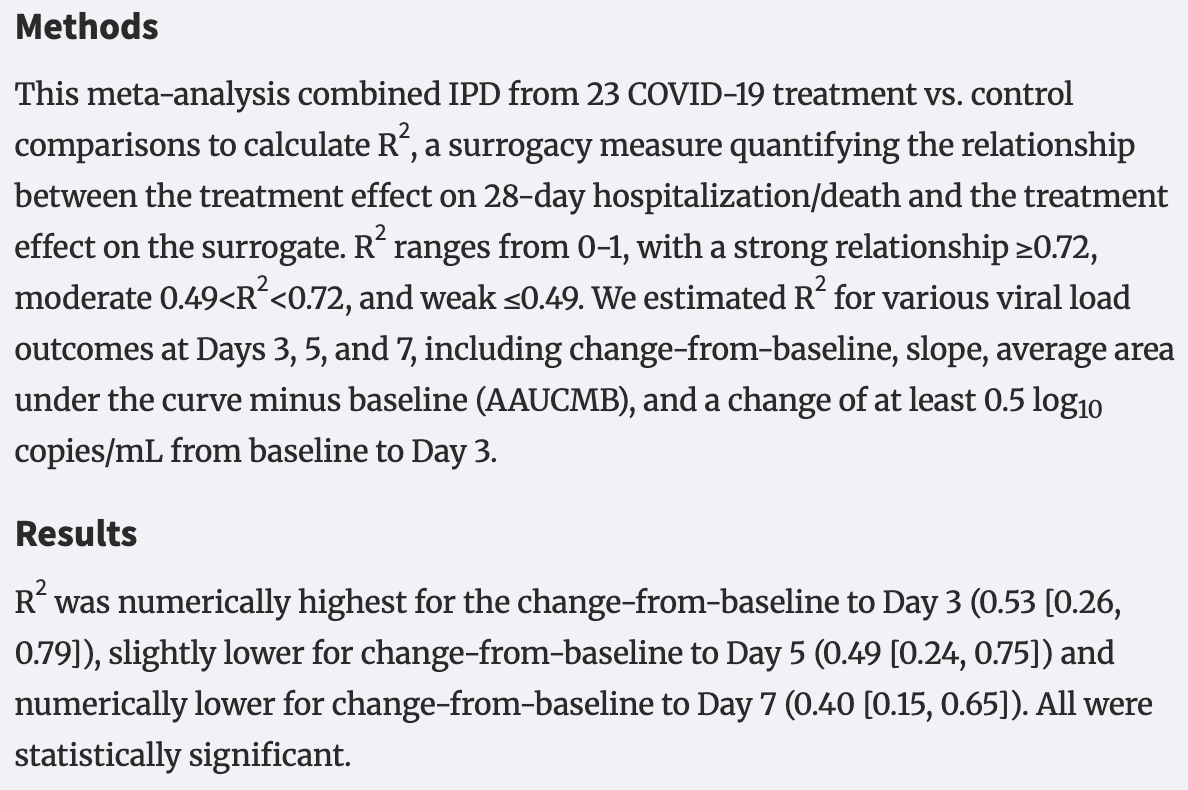
Meta-analysis of individual participant data from 23 COVID-19 studies confirming early viral load changes as surrogate endpoints for 28-day hospitalization or death, with the change in log10(viral load) from baseline to day 3 showing the strongest correlation. Day 5 changes also showed moderate correlation, day 7 changes were weaker, and more complex viral load calculations like slope or area under the curve minus baseline offered no advantage.
Mateja et al., 3 Jun 2025, peer-reviewed, 22 authors.
DOI record:
{
"DOI": "10.1093/infdis/jiaf282",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiaf282",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Virologic endpoints are used in Phase 2 trials for COVID-19 therapeutics, but they have not been established as surrogates for clinical endpoints. No meta-analysis using individual participant data (IPD) has been undertaken to identify viral load outcomes for which treatment effects are best associated with effects on hospitalization/death.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This meta-analysis combined IPD from 23 COVID-19 treatment vs. control comparisons to calculate R2, a surrogacy measure quantifying the relationship between the treatment effect on 28-day hospitalization/death and the treatment effect on the surrogate. R2 ranges from 0-1, with a strong relationship ≥0.72, moderate 0.49&lt;R2&lt;0.72, and weak ≤0.49. We estimated R2 for various viral load outcomes at Days 3, 5, and 7, including change-from-baseline, slope, average area under the curve minus baseline (AAUCMB), and a change of at least 0.5 log10 copies/mL from baseline to Day 3.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>R2 was numerically highest for the change-from-baseline to Day 3 (0.53 [0.26, 0.79]), slightly lower for change-from-baseline to Day 5 (0.49 [0.24, 0.75]) and numerically lower for change-from-baseline to Day 7 (0.40 [0.15, 0.65]). All were statistically significant.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Discussion</jats:title>\n <jats:p>Our study is the first to use IPD, allowing us to evaluate viral load collected on various study days as a surrogate to clinical outcomes. Change in log10(viral load) from baseline to Day 3 or Day 5 are moderate surrogates for 28-day hospitalization/death and suitable primary endpoints in Phase 2 clinical trials and are preferred over change-from-baseline to Day 7. Slope and AAUCMB require more calculation but didn’t improve prediction so aren’t recommended.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "https://orcid.org/0000-0002-2810-478X",
"affiliation": [
{
"name": "Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research , Frederick, MD ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Mateja",
"given": "Allyson",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research , Frederick, MD ,",
"place": [
"USA"
]
}
],
"family": "Chu",
"given": "Eric",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2769-4957",
"affiliation": [
{
"name": "Division of Biostatistics and Health Data Science, School of Public Health, University of Minnesota , Minneapolis, MN ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Murray",
"given": "Thomas A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "General Internal Medicine, Department of Medicine, University of Minnesota Medical School , Minneapolis, MD ,",
"place": [
"USA"
]
}
],
"family": "Bramante",
"given": "Carolyn T",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5601-9112",
"affiliation": [
{
"name": "Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health , Boston, MA ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Moser",
"given": "Carlee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "GlaxoSmithKline , Stevenage ,",
"place": [
"United Kingdom"
]
}
],
"family": "Givens",
"given": "Naomi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5192-8659",
"affiliation": [
{
"name": "Gilead Sciences, Inc. , Foster City, CA ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Abdelghany",
"given": "Mazin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences, Inc. , Foster City, CA ,",
"place": [
"USA"
]
}
],
"family": "Blair",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences, Inc. , Foster City, CA ,",
"place": [
"USA"
]
}
],
"family": "Chen",
"given": "Shuguang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Redwood AI , Vancouver, BC ,",
"place": [
"Canada"
]
}
],
"family": "Lat",
"given": "Prince Kumar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Redwood AI , Vancouver, BC ,",
"place": [
"Canada"
]
}
],
"family": "Harari",
"given": "Ofir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Eli Lilly and Co , Indianapolis, IN ,",
"place": [
"USA"
]
}
],
"family": "Kallewaard",
"given": "Nicole L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Eli Lilly and Co , Indianapolis, IN ,",
"place": [
"USA"
]
}
],
"family": "Macpherson",
"given": "Lisa Farmer",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4715-0060",
"affiliation": [
{
"name": "Department of Medicine, University of Minnesota Medical School , Minneapolis, MD ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Boulware",
"given": "David R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Skin Neglected Tropical Diseases and Sexually Transmitted Infection section, Fight Infectious Diseases Foundation, University Hospital Germans Trias i Pujol , Badalona ,",
"place": [
"Spain"
]
},
{
"name": "ISGlobal, Hospital Clínic - Universitat de Barcelona , Barcelona ,",
"place": [
"Spain"
]
}
],
"family": "Suñer",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Skin Neglected Tropical Diseases and Sexually Transmitted Infection section, Fight Infectious Diseases Foundation, University Hospital Germans Trias i Pujol , Badalona ,",
"place": [
"Spain"
]
},
{
"name": "Infectious Diseases Department, Universitat de Vic-Universitat Central de Catalunya , Vic ,",
"place": [
"Spain"
]
}
],
"family": "Mitjà",
"given": "Oriol",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4590-5175",
"affiliation": [
{
"name": "The Foundation for the National Institutes of Health , North Bethesda, MD ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Adam",
"given": "Stacey J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbert Wertheim School of Public Health and Human Longevity Science, University of California , San Diego, La Jolla, CA ,",
"place": [
"USA"
]
}
],
"family": "De Gruttola",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard TH Chan School of Public Health , Boston, MA ,",
"place": [
"USA"
]
}
],
"family": "Hughes",
"given": "Michael D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring , MD ,",
"place": [
"USA"
]
}
],
"family": "Rubin",
"given": "Daniel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3603-1733",
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases and Global Public Health, University of California , San Diego, La Jolla, CA ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Smith",
"given": "Davey M",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6667-6992",
"affiliation": [
{
"name": "Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health , Bethesda, MD ,",
"place": [
"USA"
]
}
],
"authenticated-orcid": false,
"family": "Potter",
"given": "Gail E",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
6,
4
]
],
"date-time": "2025-06-04T03:19:26Z",
"timestamp": 1749007166000
},
"deposited": {
"date-parts": [
[
2025,
6,
4
]
],
"date-time": "2025-06-04T03:19:26Z",
"timestamp": 1749007166000
},
"indexed": {
"date-parts": [
[
2025,
6,
4
]
],
"date-time": "2025-06-04T03:40:09Z",
"timestamp": 1749008409257,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
6,
3
]
]
},
"language": "en",
"link": [
{
"URL": "https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiaf282/63430824/jiaf282.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiaf282/63430824/jiaf282.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
6,
3
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
3
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiaf282/8156441"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The choice of viral load endpoint in early phase trials of COVID-19 treatments aiming to reduce 28-day hospitalization and/or death",
"type": "journal-article"
}