Hen egg white bovine colostrum supplement reduces symptoms of mild/moderate COVID-19: a randomized control trial
et al., Future Science OA, doi:10.2144/fsoa-2023-0024, DOH-27-062021-9191, Jul 2023
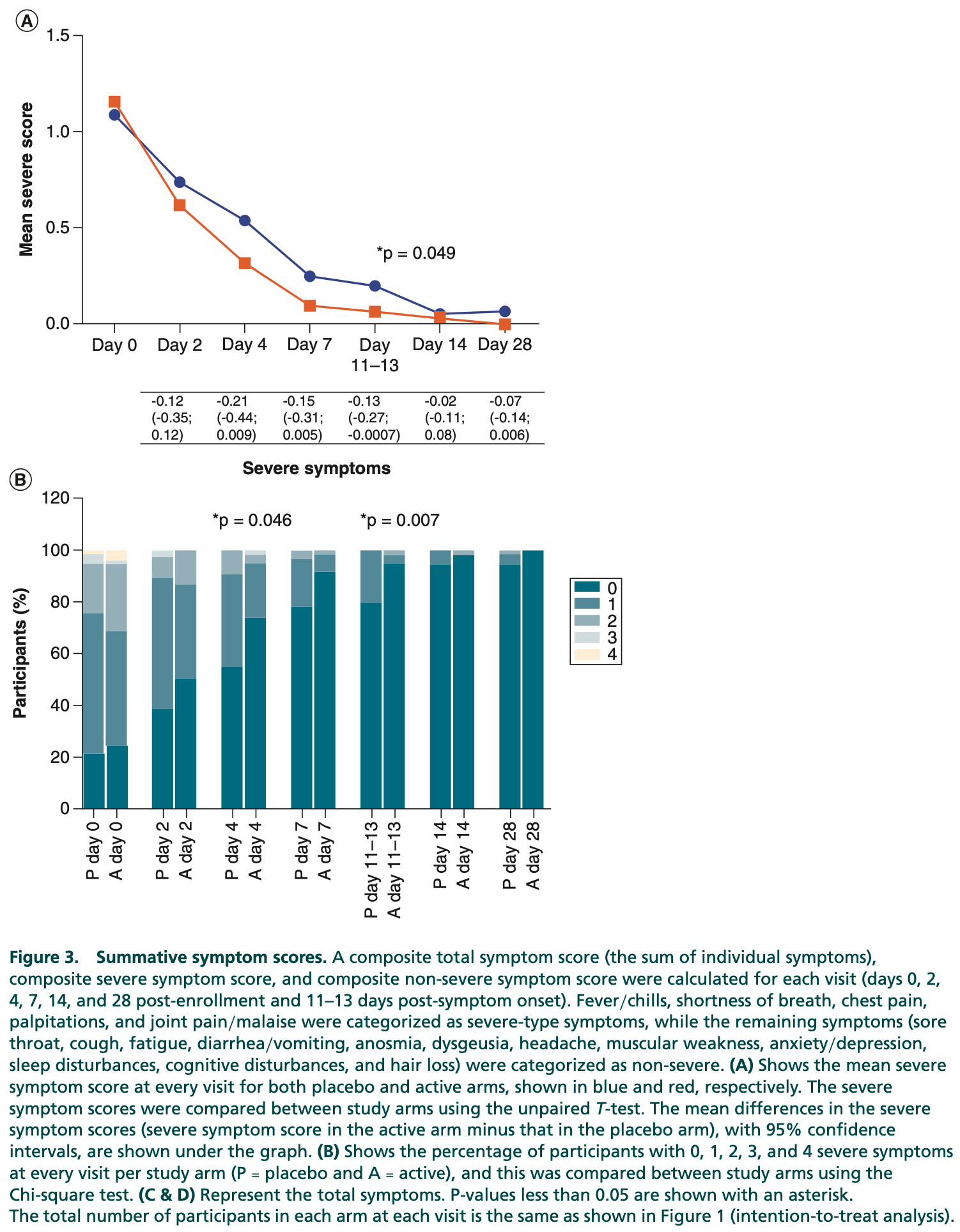
RCT 156 mild/moderate COVID-19 patients, 77 treated with hen egg white and bovine colostrum, showing faster recovery of severe symptoms with treatment. There were no significant differences in overall symptom duration, viral clearance, or post-COVID symptoms. Only one participant progressed to severe COVID-19.
|
risk of death, 202.6% higher, RR 3.03, p = 0.49, treatment 1 of 77 (1.3%), control 0 of 79 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of severe case, 202.6% higher, RR 3.03, p = 0.49, treatment 1 of 77 (1.3%), control 0 of 79 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 48.7% lower, RR 0.51, p = 1.00, treatment 1 of 77 (1.3%), control 2 of 79 (2.5%), NNT 81, COVID-19.
|
|
risk of hospitalization, 48.7% lower, RR 0.51, p = 0.68, treatment 2 of 77 (2.6%), control 4 of 79 (5.1%), NNT 41, all cause.
|
|
relative symptom score, 25.8% better, RR 0.74, p = 0.24, treatment 77, control 79, mid-recovery, day 7.
|
|
relative symptom score, 60.0% better, RR 0.40, p = 0.047, treatment 77, control 79, day 28.
|
|
relative symptom score, 2.3% better, RR 0.98, p = 0.91, treatment 77, control 79, day 14.
|
|
relative symptom score, 18.4% better, RR 0.82, p = 0.40, treatment 77, control 79, day 11-13.
|
|
relative symptom score, 4.4% better, RR 0.96, p = 0.75, treatment 77, control 79, day 4.
|
|
risk of no viral clearance, 11.2% higher, RR 1.11, p = 0.36, treatment 49 of 60 (81.7%), control 36 of 49 (73.5%), day 11-3.
|
|
risk of long COVID, 7.6% lower, RR 0.92, p = 0.84, treatment 15 of 67 (22.4%), control 16 of 66 (24.2%), NNT 54, day 42.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mann et al., 20 Jul 2023, Double Blind Randomized Controlled Trial, placebo-controlled, South Africa, peer-reviewed, 14 authors, study period 28 July, 2021 - 5 July, 2022, this trial uses multiple treatments in the treatment arm (combined with bovine colostrum and egg white) - results of individual treatments may vary, trial DOH-27-062021-9191.
Contact: mannj@ukzn.ac.za.
Hen egg white bovine colostrum supplement reduces symptoms of mild/moderate COVID-19: a randomized control trial
Future Science OA, doi:10.2144/fsoa-2023-0024
The ability of a hen egg white bovine colostrum supplement to prevent severe COVID-19 was tested in a double-blind randomized control study. Methods: Adults with mild/moderate COVID-19, risk factors for severe disease, and within 5 days of symptom onset were assigned to the intervention (n = 77) or placebo (n = 79) arms. Symptoms were documented until day 42 post-enrollment and viral clearance was assessed at 11-13 days post-symptom onset. Results: One participant developed severe COVID-19. The severe-type symptom score was lower in the active arm at 11-13 days post-symptom onset (p = 0.049). Chest pain, fever/chills, joint pain/malaise, and sore throat were significantly less frequent in the active arm. No differences in viral clearance were observed. Conclusion: The intervention reduced symptoms of mild/moderate COVID-19. Clinical Trial Registration: DOH-27-062021-9191 (South African National Clinical Trials Register) Plain language summary: Natural proteins found in milk (lactoferrin) and egg white (ovotransferrin and lysozyme) could have therapeutic value in COVID-19 through their effects on the immune system. We identified bovine colostrum and hen egg white powders containing adequate quantities of these proteins. We investigated whether short-term daily consumption of a hen egg white and bovine colostrum mixture (reconstituted with glycerin and water) could reduce the risk of progression to severe disease and assist in the recovery of patients with mild or moderate COVID-19. Adults with mild or moderate COVID-19 who were within 5 days of symptom onset and had risk factors for severe disease were enrolled, and randomly assigned to take a hen egg white and bovine colostrum mixture or placebo mixture twice daily for 5 days, and then followed up telephonically for 6 weeks. The main findings were that consumption of the hen egg white and bovine colostrum mixture was associated with fewer protocol-defined severe-type symptoms overall, and in particular lower frequencies of joint pain/malaise, chest pain, fever/chills, and sore throat. Only one individual developed severe COVID-19 and therefore the effect of the intervention on reducing the risk of progression to severe disease could not be assessed. The results of this study suggest that consumption of the hen egg white bovine colostrum mixture within a few days of symptom onset lessens symptoms in people with mild or moderate COVID-19.
Ethical conduct of research The protocol was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BREC 00002220/2020). In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement The authors certify that this manuscript reports original clinical trial data. Deidentified individual participant data that underlie the results reported in the article are available in Mendeley data (https://data.mendeley.com/datasets/79wzs93pf3/1). It is currently available with no foreseeable end date.
Open access This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecomm ons.org/licenses/by/4.0/
Summary points • Ovotransferrin and lysozyme in hen egg white and lactoferrin in bovine colostrum have immunomodulatory and antiviral properties. • It was hypothesized that consumption of a hen egg white bovine colostrum mixture in early infection would reduce the risk of progression to severe disease and assist in the recovery of individuals with mild/moderate COVID-19. • In this double-blind randomized control study, COVID-19-positive individuals at increased risk of severe disease and within 5 days of symptom onset were randomly assigned to consume a hen egg white bovine colostrum mixture (n = 77) or placebo (n = 79) twice daily for 5 days. • Only 1 participant (active arm) progressed to severe..
References
Abdullah, Myers, Basu, Decreased severity of disease during the first global omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa, Int. J. Infect. Dis
Abeyrathne, Huang, Ahn, Antioxidant, angiotensin-converting enzyme inhibitory activity and other functional properties of egg white proteins and their derived peptides -A review, Poult. Sci
Actor, Hwang, Kruzel, Lactoferrin as a Natural Immune Modulator, Curr. Pharm. Des
Ashraf, Ashraf, Ashraf, Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): a multicenter placebo-controlled randomized clinical trial, Phytother. Res
Berlutti, Pantanella, Natalizi, Antiviral Properties of Lactoferrin-A Natural Immunity Molecule, Molecules
Bhavsar, Liu, Hardej, Liu, Cantor, Aerosolized recombinant human lysozyme ameliorates Pseudomonas aeruginosa-induced pneumonia in hamsters, Exp. Lung Res
Bol'shakova, Medvedeva, Zhuravleva, Lysozyme in the feeding of premature infants with mixed pathology, Antibiotiki
Campione, Lanna, Cosio, Lactoferrin as antiviral treatment in COVID-19 management: preliminary evidence, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph182010985
Chiurciu, Chiurciu, Oporanu, PC2 ovotransferrin: characterization and alternative immunotherapeutic activity, Evid. Based Complement. Alternat. Med, doi:10.1155/2017/8671271
Council, Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA, Nephrol. Dial. Transplant
Cutone, Lepanto, Rosa, Aerosolized bovine lactoferrin counteracts infection, inflammation and iron dysbalance in a cystic fibrosis mouse model of Pseudomonas aeruginosa chronic lung infection, Int. J. Mol. Sci
D'ecclesiis, Gavioli, Martinoli, Vitamin D and SARS-CoV2 infection, severity and mortality: a systematic review and meta-analysis, PLOS ONE
De Douder, Morias, On lysozyme therapy. I. Lysozyme tablets in treatment of some localized and generalized viral skin diseases, Medickon
Fernández-Musoles, Salom, Martínez-Maqueda, López-Díez, Recio et al., Antihypertensive effects of lactoferrin hydrolyzates: inhibition of angiotensin-and endothelin-converting enzymes, Food Chem
Fond, Nemani, Etchecopar-Etchart, Association between mental health disorders and mortality among patients with COVID-19 in 7 Countries: a systematic review and meta-analysis, JAMA Psychiatry
Fontanet, Autran, Lina, Kieny, Karim et al., SARS-CoV-2 variants and ending the COVID-19 pandemic, Lancet
Gandhi, Lynch, Rio, Mild or Moderate Covid-19, N. Engl. J. Med
Gao, Piernas, Astbury, Associations between body-mass index and COVID-19 severity in 6•9 million people in England: a prospective, community-based, cohort study, Lancet Diabetes Endocrinol
Giansanti, Massucci, Giardi, Antiviral activity of ovotransferrin derived peptides, Biochem. Biophys. Res. Commun
Jiao, Zhang, Lin, Gao, Zhang, The Ovotransferrin-Derived Peptide IRW Attenuates Lipopolysaccharide-Induced Inflammatory Responses, Biomed. Res. Int, doi:10.1155/2019/8676410
Jin, Yang, Virology, epidemiology, pathogenesis, and control of COVID-19, Viruses
Kaito, Iwasa, Fujita, Effect of lactoferrin in patients with chronic hepatitis C: combination therapy with interferon and ribavirin, J. Gastroenterol. Hepatol
Kang, Kong, Body mass index and severity/fatality from coronavirus disease 2019: a nationwide epidemiological study in Korea, PLOS ONE
Kim, Moon, Ahn, Paik, Park, Antioxidant effects of ovotransferrin and its hydrolysates, Poult. Sci
Kompaniyets, Pennington, Goodman, Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, Prev. Chronic. Dis
Kruzel, Zimecki, Actor, Lactoferrin in a Context of Inflammation-Induced Pathology, Front. Immunol
Kuehn, More Severe Obesity Leads to More Severe COVID-19 in Study, JAMA
Kumar, Abdulrahman, Alali, Otoom, Atkin et al., Time till viral clearance of severe acute respiratory syndrome coronavirus 2 is similar for asymptomatic and non-critically symptomatic individuals, Front. Med. (Lausanne), doi:10.3389/fmed.2021.616927
Lee, Kovacs-Nolan, Archbold, Therapeutic potential of hen egg white peptides for the treatment of intestinal inflammation, J. Funct. Foods
Lee, Kovacs-Nolan, Yang, Archbold, Fan et al., Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis, J. Agric. Food Chem
Lepanto, Rosa, Paesano, Valenti, Cutone, • The ability of lactoferrin to act as an immune "sensing" agent is described, and the evidence for reduction of immune pathology in a wide range of disease models following lactoferrin administration is reviewed, Molecules
Li, Sun, Han, Ying, Wang, A study on the predictors of disease severity of COVID-19, Med. Sci. Monit
Liao, Fan, Wu, Egg white-derived antihypertensive peptide IRW (Ile-Arg-Trp) inhibits angiotensin II-stimulated migration of vascular smooth muscle cells via angiotensin type I receptor, J. Agric. Food Chem
Luniakin, Bogomaz, Lysozyme in the overall treatment of children with an influenza infection and pneumonia, Pediatr. Akus. Ginekol, doi:futuresciencegroup10.2144/fsoa-2023-0024
Madhi, Kwatra, Myers, Population Immunity and COVID-19 Severity with Omicron Variant in South Africa, N. Engl. J. Med
Majumder, Chakrabarti, Davidge, Wu, Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress, J. Agric. Food Chem
Mann, Ndung, The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immune-modulating agents in COVID-19, Future Virol, doi:10.2217/fvl-2020-0170
Mcmahan, Giffin, Tostanoski, Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters, Med
Mirabelli, Wotring, Zhang, Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19, PNAS
Moon, Lee, Kim, Paik, Ahn, In vitro cytotoxic and ACE-inhibitory activities of promod 278P hydrolysate of ovotransferrin from chicken egg white, Poult. Sci
Mulder, Connellan, Oliver, Morris, Stevenson, Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males, Nutr. Res
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat. Med
Nimalaratne, Bandara, Wu, Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white, Food Chem
Oakes, Kernberg, Carter, Pregnancy as a risk factor for severe coronavirus disease 2019 using standardized clinical criteria, Am. J. Obstet. Gynecol. MFM, doi:10.1016/j.ajogmf.2021.100319
Oe, Sakamoto, Nishiyama, Egg white hydrolyzate reduces mental fatigue: randomized, double-blind, controlled study, BMC Res. Notes
Péus, Newcomb, Hofer, Appraisal of the Karnofsky performance status and proposal of a simple algorithmic system for its evaluation, BMC Med. Inform. Decis. Mak
Ragland, Criss, From bacterial killing to immune modulation: recent insights into the functions of lysozyme, PLoS Pathog
Ronghui, The reasearch on the anti-fatigue effect of whey protein powder in basketball training, Open Biomed. Eng. J
Rosa, Tripepi, Naldi, Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study, J. Clin. Med
Rubbini, Rizzi, Ruozi, Use of lysozyme in parodontopathy, Controlled clinical trials. Riv. It. Biol. Med
Rutherfurd, Gill, Peptides affecting coagulation, Br. J. Nutr
Salama, Schaalan, A pilot study on the effect of lactoferrin on Alzheimer's disease pathological sequelae: impact of the p-Akt/PTEN pathway, Biomed. Pharmacother
Sandoval, Nguyen, Vahidy, Graviss, Risk factors for severity of COVID-19 in hospital patients age 18-29 years, PLOS ONE
Sato, Oe, Nakano, Kawasaki, Hirayama, A random controlled study of the prophylactic effect of lysozyme chloride on post-transfusion hepatitis, Hepatogastroenterology
Sava, Pharmacological aspects and therapeutic applications of lysozymes, Lysozymes: Model Enzymes in Biochemistry and Biology. Jolles P
Swanstrom, Schinazi, Lethal mutagenesis as an antiviral strategy, Science
Tagashira, Nishi, Matsumoto, Sugahara, Anti-inflammatory effect of lysozyme from hen egg white on mouse peritoneal macrophages, Cytotechnology
Tagashira, Nishi, Sugahara, Lysozyme from hen egg white ameliorates lipopolysaccharide-induced systemic inflammation in mice, Cytotechnology
Togawa, Nagase, Tanaka, Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance, J. Gastroenterol. Hepatol
Wakabayashi, Oda, Yamauchi, Abe, Lactoferrin for prevention of common viral infections, J. Infect. Chemother
Who, Clinical management of COVID-19
Who, WHO Coronavirus (COVID-19) Dashboard
Wisgrill, Wessely, Spittler, Förster-Waldl, Berger et al., Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages, Clin. Exp. Immunol
Wotring, Fursmidt, Ward, Sexton, Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern, J. Dairy Sci
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Yu, Yin, Zhao, Chen, Liu, Antihypertensive effect of angiotensin-converting enzyme inhibitory peptide RVPSL on spontaneously hypertensive rats by regulating gene expression of the renin-angiotensin system, J. Agric. Food Chem
Zerbo, Lewis, Fireman, Population-based assessment of risks for severe COVID-19 disease outcomes, Influenza Other Respir. Viruses
Zhang, Cytokines, inflammation, and pain, Int. Anesthesiol. Clin
DOI record:
{
"DOI": "10.2144/fsoa-2023-0024",
"ISSN": [
"2056-5623"
],
"URL": "http://dx.doi.org/10.2144/fsoa-2023-0024",
"abstract": "<jats:p> Aim: The ability of a hen egg white bovine colostrum supplement to prevent severe COVID-19 was tested in a double-blind randomized control study. Methods: Adults with mild/moderate COVID-19, risk factors for severe disease, and within 5 days of symptom onset were assigned to the intervention (n = 77) or placebo (n = 79) arms. Symptoms were documented until day 42 post-enrollment and viral clearance was assessed at 11–13 days post-symptom onset. Results: One participant developed severe COVID-19. The severe-type symptom score was lower in the active arm at 11–13 days post-symptom onset (p = 0.049). Chest pain, fever/chills, joint pain/malaise, and sore throat were significantly less frequent in the active arm. No differences in viral clearance were observed. Conclusion: The intervention reduced symptoms of mild/moderate COVID-19. </jats:p><jats:p> Clinical Trial Registration: DOH-27-062021-9191 (South African National Clinical Trials Register) </jats:p>",
"alternative-id": [
"10.2144/fsoa-2023-0024"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2398-1273",
"affiliation": [
{
"name": "HIV Pathogenesis Programme, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"authenticated-orcid": false,
"family": "Mann",
"given": "Jaclyn Kelly",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Biostatistics Research Unit, South African Medical Research Council, Durban, 4091, South Africa"
}
],
"family": "Reddy",
"given": "Tarylee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HIV Pathogenesis Programme, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "der Stok",
"given": "Mary van",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HIV Pathogenesis Programme, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "Ngubane",
"given": "Ayanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HIV Pathogenesis Programme, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "Mulaudzi",
"given": "Takalani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biostatistics Research Unit, South African Medical Research Council, Durban, 4091, South Africa"
}
],
"family": "Mchunu",
"given": "Nobuhle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biostatistics Research Unit, South African Medical Research Council, Durban, 4091, South Africa"
}
],
"family": "Nevhungoni",
"given": "Portia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "Manickchund",
"given": "Nithendra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "Manickchund",
"given": "Pariva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "Louise Cairns",
"given": "Chelline Helena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aurum Institute, Johannesburg, 2194, South Africa"
}
],
"family": "Govender",
"given": "Vaneshree",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HIV Pathogenesis Programme, University of KwaZulu-Natal, Durban, 4001, South Africa"
},
{
"name": "Africa Health Research Institute, Durban, 4001, South Africa"
},
{
"name": "Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology & Harvard University, Cambridge, MA 02139, USA"
},
{
"name": "Division of Infection & Immunity, University College London, London, WC1E 6BT, UK"
}
],
"family": "Ndung'u",
"given": "Thumbi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "Suleman Moosa",
"given": "Mahomed Yunus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, University of KwaZulu-Natal, Durban, 4001, South Africa"
}
],
"family": "Gosnell",
"given": "Bernadett Isabel",
"sequence": "additional"
}
],
"container-title": "Future Science OA",
"container-title-short": "Future Science OA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
20
]
],
"date-time": "2023-07-20T10:40:01Z",
"timestamp": 1689849601000
},
"deposited": {
"date-parts": [
[
2023,
7,
20
]
],
"date-time": "2023-07-20T10:40:17Z",
"timestamp": 1689849617000
},
"funder": [
{
"DOI": "10.13039/501100011858",
"award": [
"SARSCoV2-3-20-006"
],
"doi-asserted-by": "crossref",
"name": "African Academy of Sciences"
},
{
"DOI": "10.13039/501100022674",
"doi-asserted-by": "crossref",
"name": "Victor Daitz Foundation"
}
],
"indexed": {
"date-parts": [
[
2023,
7,
21
]
],
"date-time": "2023-07-21T04:25:16Z",
"timestamp": 1689913516209
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
7,
20
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.future-science.com/doi/pdf/10.2144/fsoa-2023-0024",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2641",
"original-title": [],
"prefix": "10.2144",
"published": {
"date-parts": [
[
2023,
7,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
20
]
]
},
"publisher": "Future Science Ltd",
"reference": [
{
"key": "B1",
"unstructured": "WHO. WHO Coronavirus (COVID-19) Dashboard (2022). https://covid19.who.int/"
},
{
"DOI": "10.3390/v12040372",
"doi-asserted-by": "publisher",
"key": "B2"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"doi-asserted-by": "publisher",
"key": "B3"
},
{
"DOI": "10.1016/S0140-6736(21)00370-6",
"doi-asserted-by": "publisher",
"key": "B4"
},
{
"DOI": "10.1126/science.abn0048",
"doi-asserted-by": "publisher",
"key": "B5"
},
{
"DOI": "10.2217/fvl-2020-0170",
"doi-asserted-by": "publisher",
"key": "B6"
},
{
"DOI": "10.3390/molecules16086992",
"doi-asserted-by": "publisher",
"key": "B7"
},
{
"DOI": "10.1155/2017/8671271",
"doi-asserted-by": "publisher",
"key": "B8"
},
{
"DOI": "10.1371/journal.ppat.1006512",
"doi-asserted-by": "publisher",
"key": "B9"
},
{
"DOI": "10.1007/978-3-0348-9225-4_22",
"doi-asserted-by": "publisher",
"key": "B10"
},
{
"DOI": "10.3390/ijms20092128",
"doi-asserted-by": "publisher",
"key": "B11"
},
{
"DOI": "10.1073/pnas.2105815118",
"doi-asserted-by": "publisher",
"key": "B12"
},
{
"DOI": "10.3382/ps/pex399",
"doi-asserted-by": "publisher",
"key": "B13"
},
{
"DOI": "10.1016/j.bbrc.2005.03.125",
"doi-asserted-by": "publisher",
"key": "B14"
},
{
"DOI": "10.1016/j.nutres.2008.05.007",
"doi-asserted-by": "publisher",
"key": "B15"
},
{
"DOI": "10.3382/ps.2012-02150",
"doi-asserted-by": "publisher",
"key": "B16"
},
{
"DOI": "10.1016/j.foodchem.2015.05.014",
"doi-asserted-by": "publisher",
"key": "B17"
},
{
"DOI": "10.3390/molecules24071323",
"doi-asserted-by": "publisher",
"key": "B18"
},
{
"DOI": "10.1111/cei.13108",
"doi-asserted-by": "publisher",
"key": "B19"
},
{
"DOI": "10.1016/j.jiac.2014.08.003",
"doi-asserted-by": "publisher",
"key": "B20"
},
{
"DOI": "10.1021/jf3046076",
"doi-asserted-by": "publisher",
"key": "B21"
},
{
"DOI": "10.1017/S0007114500002312",
"doi-asserted-by": "publisher",
"key": "B22"
},
{
"DOI": "10.1016/j.foodchem.2012.12.049",
"doi-asserted-by": "publisher",
"key": "B23"
},
{
"DOI": "10.3382/ps/pew459",
"doi-asserted-by": "publisher",
"key": "B24"
},
{
"DOI": "10.1021/acs.jafc.8b00483",
"doi-asserted-by": "publisher",
"key": "B25"
},
{
"DOI": "10.1111/j.1440-1746.2007.04858.x",
"doi-asserted-by": "publisher",
"key": "B26"
},
{
"author": "Luniakin AA",
"first-page": "11",
"journal-title": "Pediatr. Akus. Ginekol.",
"key": "B27",
"volume": "1",
"year": "1977"
},
{
"author": "Sato M",
"first-page": "135",
"journal-title": "Hepatogastroenterology",
"key": "B28",
"volume": "28",
"year": "1981"
},
{
"author": "De Douder C",
"first-page": "19",
"journal-title": "Medickon",
"key": "B29",
"volume": "3",
"year": "1974"
},
{
"DOI": "10.1046/j.1440-1746.2002.02868.x",
"doi-asserted-by": "publisher",
"key": "B30"
},
{
"DOI": "10.2174/138161209788453202",
"doi-asserted-by": "publisher",
"key": "B31"
},
{
"DOI": "10.1021/jf803133b",
"doi-asserted-by": "publisher",
"key": "B32"
},
{
"author": "Rubbini A",
"first-page": "45",
"journal-title": "Riv. It. Biol. Med.",
"key": "B33",
"volume": "9",
"year": "1989"
},
{
"DOI": "10.3109/01902140903154608",
"doi-asserted-by": "publisher",
"key": "B34"
},
{
"author": "Bol'shakova AM",
"first-page": "784",
"journal-title": "Antibiotiki",
"key": "B35",
"volume": "29",
"year": "1984"
},
{
"DOI": "10.1155/2019/8676410",
"doi-asserted-by": "publisher",
"key": "B36"
},
{
"DOI": "10.1056/NEJMcp2009249",
"doi-asserted-by": "publisher",
"key": "B37"
},
{
"DOI": "10.1371/journal.pone.0255544",
"doi-asserted-by": "publisher",
"key": "B38"
},
{
"DOI": "10.1093/ndt/gfaa314",
"doi-asserted-by": "publisher",
"key": "B39"
},
{
"DOI": "10.1371/journal.pone.0253640",
"doi-asserted-by": "publisher",
"key": "B40"
},
{
"DOI": "10.1016/S2213-8587(21)00089-9",
"doi-asserted-by": "publisher",
"key": "B41"
},
{
"author": "Kuehn BM",
"first-page": "1603",
"issue": "16",
"journal-title": "JAMA",
"key": "B42",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/j.ajogmf.2021.100319",
"doi-asserted-by": "publisher",
"key": "B43"
},
{
"DOI": "10.5888/pcd18.210123",
"doi-asserted-by": "publisher",
"key": "B44"
},
{
"DOI": "10.1001/jamapsychiatry.2021.2274",
"doi-asserted-by": "publisher",
"key": "B45"
},
{
"DOI": "10.1111/irv.12901",
"doi-asserted-by": "publisher",
"key": "B46"
},
{
"key": "B47",
"unstructured": "WHO. Clinical management of COVID-19 (2020). https://www.who.int/teams/health-care-readiness/covid-19"
},
{
"DOI": "10.12659/MSM.927167",
"doi-asserted-by": "publisher",
"key": "B48"
},
{
"DOI": "10.1186/1472-6947-13-72",
"doi-asserted-by": "publisher",
"key": "B49"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "B50"
},
{
"DOI": "10.3389/fimmu.2017.01438",
"doi-asserted-by": "publisher",
"key": "B51"
},
{
"DOI": "10.1007/s10616-019-00296-4",
"doi-asserted-by": "publisher",
"key": "B52"
},
{
"DOI": "10.1007/s10616-017-0184-2",
"doi-asserted-by": "publisher",
"key": "B53"
},
{
"DOI": "10.1097/AIA.0b013e318034194e",
"doi-asserted-by": "publisher",
"key": "B54"
},
{
"DOI": "10.1186/s13104-020-05288-8",
"doi-asserted-by": "publisher",
"key": "B55"
},
{
"DOI": "10.2174/1874120701509010330",
"doi-asserted-by": "publisher",
"key": "B56"
},
{
"DOI": "10.1016/j.ijid.2021.12.357",
"doi-asserted-by": "publisher",
"key": "B57"
},
{
"DOI": "10.1056/NEJMoa2119658",
"doi-asserted-by": "publisher",
"key": "B58"
},
{
"DOI": "10.1016/j.medj.2022.03.004",
"doi-asserted-by": "publisher",
"key": "B59"
},
{
"DOI": "10.3168/jds.2021-21247",
"doi-asserted-by": "publisher",
"key": "B60"
},
{
"DOI": "10.3390/ijerph182010985",
"doi-asserted-by": "publisher",
"key": "B61"
},
{
"DOI": "10.3390/jcm10184276",
"doi-asserted-by": "publisher",
"key": "B62"
},
{
"DOI": "10.1371/journal.pone.0268396",
"author": "D'Ecclesiis O",
"doi-asserted-by": "crossref",
"first-page": "e0268396",
"issue": "7",
"journal-title": "PLOS ONE",
"key": "B63",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7640",
"doi-asserted-by": "publisher",
"key": "B64"
},
{
"DOI": "10.1021/jf405189y",
"doi-asserted-by": "publisher",
"key": "B65"
},
{
"DOI": "10.1016/j.jff.2009.01.005",
"doi-asserted-by": "publisher",
"key": "B66"
},
{
"DOI": "10.1016/j.biopha.2018.12.118",
"doi-asserted-by": "publisher",
"key": "B67"
},
{
"author": "Kumar N",
"journal-title": "Front. Med. (Lausanne)",
"key": "B68",
"volume": "8",
"year": "2021"
}
],
"reference-count": 68,
"references-count": 68,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.future-science.com/doi/10.2144/fsoa-2023-0024"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Biotechnology"
],
"subtitle": [],
"title": "Hen egg white bovine colostrum supplement reduces symptoms of mild/moderate COVID-19: a randomized control trial",
"type": "journal-article"
}