Impact of Elevated Liver Enzymes on the Severity of Clinical Course of COVID‐19: A Retrospective Study From Saudi Arabia
et al., International Journal of Hepatology, doi:10.1155/ijh/7385050, Jan 2025
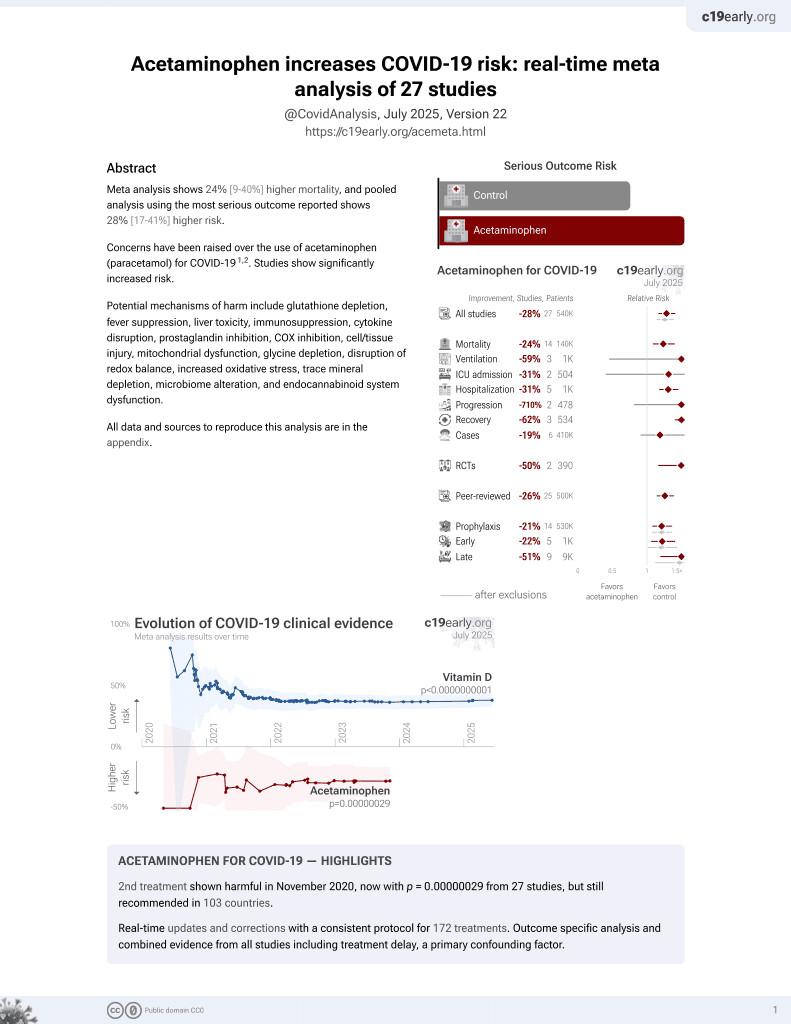
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
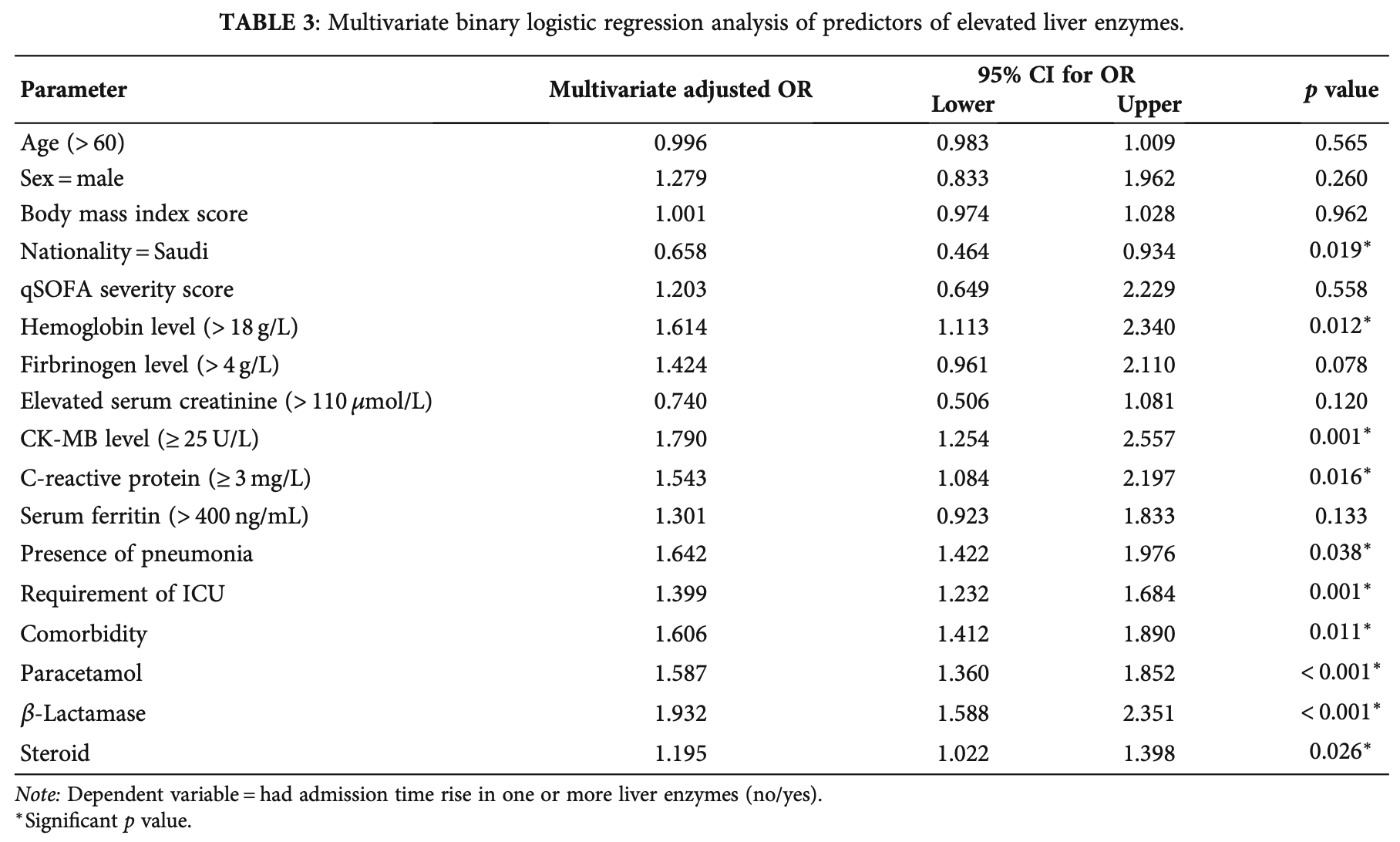
Retrospective 1,033 hospitalized COVID-19 patients in Saudi Arabia showing significantly higher risk of elevated liver enzymes with paracetamol, beta-lactam antibiotics, and steroids.
Paracetamol is also known as acetaminophen, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
Makkawy et al., 31 Jan 2025, retrospective, Saudi Arabia, peer-reviewed, mean age 49.9, 7 authors, study period March 2020 - October 2020.
Contact: eamakkawy@gmail.com.
Impact of Elevated Liver Enzymes on the Severity of Clinical Course of COVID‐19: A Retrospective Study From Saudi Arabia
International Journal of Hepatology, doi:10.1155/ijh/7385050
Background: According to recent research, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause liver injury, which may be linked to a poorer prognosis. In this retrospective study, we evaluated the incidence of high liver enzyme levels in patients with COVID-19 and their correlation with the severity and prognosis of clinical outcomes. Methods: A retrospective cohort study was performed from March 2020 to October 2020 at Prince Mohammed Bin Abdulaziz Hospital (PMAH), Riyadh, Saudi Arabia. Demographic information of COVID-19 patients as well as data on clinical features and laboratory parameters were collected. Pearson's correlation (r) test was used to assess the correlation between elevated liver enzymes and COVID-19 severity. The multivariate logistic binary regression analysis was used to identify the predictors of elevated liver enzyme levels and mortality among patients with COVID-19. Results: This cohort included 1033 patients, 73% of whom were male, with a mean age of 49.9 years. Elevated liver enzymes were observed in 52.7% of patients, most commonly with a hepatitis pattern (63.1%). Elevated levels of hemoglobin, creatine kinasemyocardial band, and C-reactive protein, as well as pneumoniae, the requirement of an intensive care unit, comorbidities, and the use of paracetamol, β-lactamase, and steroids were significant predictors of elevated liver enzymes (p < 0 05). Interestingly, Saudi patients (p = 0 019) were found to be a significant protective predictor of elevated liver enzymes. Our findings revealed that elevated liver enzyme levels were significantly correlated with the severity of COVID-19 (p < 0 05) in terms of qSOFA score. Moreover, older age, diabetes, qSOFA score, and elevated hepatitis enzymes were associated with mortality (p = 0 043). Conclusions: Elevated liver enzyme levels were common in patients with COVID-19 and were associated with the severity and prognosis of clinical outcomes.
Conflicts of Interest The authors declare no conflicts of interest.
Author Contributions Eyad Makkawy: writingreview and editing. Shaden Mohammed Alamro: methodology, resources. Safiya Ibn Awadh: data curation. Reem Basalasil: data curation. Ziyad Alkhelaiwi: data curation. Bassam AL-Mutairi: data curation. Fatimah Rebh: formal analysis, writingreview and editing.
Supporting Information Additional supporting information can be found online in the Supporting Information section. (Supporting Information) Table S1 reported data about the type of comorbidity, symptoms, medication use after admission, and medical complications of the COVID-19 patients.
References
Almalki, Alahmari, Alajlan, Continuity of Care in Primary Healthcare Settings Among Patients With Chronic Diseases in Saudi Arabia, SAGE Open Medicine, doi:10.1177/20503121231208648
Anastasiou, Korth, Herbstreit, Witzke, Lange, Mild Versus Severe Liver Injury in SARS-CoV-2 Infection, Digestive Diseases, doi:10.1159/000510758
Boettler, Newsome, Mondelli, Care of Patients With Liver Disease During the COVID-19 Pandemic: EASL-ESCMID Position Paper, JHEP Reports, doi:10.1016/j.jhepr.2020.100113
Cai, Huang, Yu, COVID-19: Abnormal Liver Function Tests, Journal of Hepatology, doi:10.1016/j.jhep.2020.04.006
Chen, Zhou, Dong, Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study, Lancet, doi:10.1016/S0140-6736(20)30211-7
Da, Suchman, Roth, Cholestatic Liver Injury in COVID-19 Is a Rare and Distinct Entity and Is Associated With Increased Mortality, Journal of Internal Medicine, doi:10.1111/joim.13292
Ding, He, Zhang, Organ Distribution of Severe Acute Respiratory Syndrome (SARS) Associated Coronavirus (SARS-CoV) in SARS Patients: Implications for Pathogenesis and Virus Transmission Pathways, Journal of Pathology, doi:10.1002/path.1560
Díaz, Idalsoaga, Cannistra, High Prevalence of Hepatic Steatosis and Vascular Thrombosis in COVID-19: A Systematic Review and Meta-Analysis of Autopsy Data, WJG, doi:10.3748/wjg.v26.i48.7693
Fu, Zhu, Bai, Clinical Features of Patients Infected With Coronavirus Disease 2019 With Elevated Liver Biochemistries: A Multicenter, Retrospective Study, Hepatology, doi:10.1002/hep.31446
Guan, Ni, Hu, Clinical Characteristics of Coronavirus Disease 2019 in China, New England Journal of Medicine, doi:10.1056/NEJMoa2002032
Higuera-De La Tijera, Servín-Caamaño, Reyes-Herrera, Impact of Liver Enzymes on SARS-CoV-2 Infection and the Severity of Clinical Course of COVID-19, Liver Research, doi:10.1016/j.livres.2021.01.001
Huang, Wang, Li, Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Kalas, Chavez, Leon, Taweesedt, Surani, Abnormal Liver Enzymes: A Review for Clinicians, WJH, doi:10.4254/wjh.v13.i11.1688
Karlafti, Paramythiotis, Pantazi, Drug-Induced Liver Injury in Hospitalized Patients During SARS-CoV-2 Infection, Medicina, doi:10.3390/medicina58121848
Kovalic, Huang, Thuluvath, Satapathy, Elevated Liver Biochemistries in Hospitalized Chinese Patients With Severe COVID-19: Systematic Review and Meta-Analysis, Hepatology, doi:10.1002/hep.31472
Kovalic, Satapathy, Thuluvath, Prevalence of Chronic Liver Disease in Patients With COVID-19 and Their Clinical Outcomes: A Systematic Review and Meta-Analysis, Hepatology International, doi:10.1007/s12072-020-10078-2
Lei, Liu, Zhou, Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China, Hepatology, doi:10.1002/hep.31301
Loganathan, Kuppusamy, Wankhar, Angiotensin-Converting Enzyme 2 (ACE2): COVID 19 Gate Way to Multiple Organ Failure Syndromes, Respiratory Physiology & Neurobiology, doi:10.1016/j.resp.2020.103548
Marjot, Webb, Barritt, COVID-19 and Liver Disease: Mechanistic and Clinical Perspectives, Nature Reviews. Gastroenterology & Hepatology, doi:10.1038/s41575-021-00426-4
Nardo, Schneeweiss-Gleixner, Bakail, Dixon, Lax et al., Pathophysiological Mechanisms of Liver Injury in COVID-19, Liver International, doi:10.1111/liv.14730
Phipps, Barraza, Lasota, Acute Liver Injury in COVID-19: Prevalence and Association With Clinical Outcomes in a Large U.S. Cohort, Hepatology, doi:10.1002/hep.31404
Picon, Joveleviths, Alvares-Da-Silva, Minimal Liver Enzymes Abnormalities at Admission Are Related to Severe COVID-19 Clinical Course in a Large Brazilian Cohort, Arquivos de Gastroenterologia, doi:10.1590/s0004-2803.202301000-03
Polyzogopoulou, Amoiridou, Abraham, Ventoulis, Acute Liver Injury in COVID-19 Patients Hospitalized in the Intensive Care Unit: Narrative Review, World Journal of Gastroenterology, doi:10.3748/wjg.v28.i47.6662
Raoult, Zumla, Locatelli, Ippolito, Kroemer, Coronavirus Infections: Epidemiological, Clinical and Immunological Features and Hypotheses, CST, doi:10.15698/cst2020.04.216
Savransky, Nanayakkara, Vivero, Chronic Intermittent Hypoxia Predisposes to Liver Injury †, Hepatology, doi:10.1002/hep.21593
Sun, Aghemo, Forner, Valenti, COVID-19 and Liver Disease, Liver International, doi:10.1111/liv.14470
Yip, Lui, Wong, Liver Injury Is Independently Associated With Adverse Clinical Outcomes in Patients With COVID-19, Gut, doi:10.1136/gutjnl-2020-321726
Zhang, Shi, Wang, Liver Injury in COVID-19: Management and Challenges, Lancet Gastroenterology & Hepatology, doi:10.1016/S2468-1253(20)30057-1
Zhu, Cai, Fan, Clinical Value of Immune-Inflammatory Parameters to Assess the Severity of Coronavirus Disease 2019, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.04.041
DOI record:
{
"DOI": "10.1155/ijh/7385050",
"ISSN": [
"2090-3448",
"2090-3456"
],
"URL": "http://dx.doi.org/10.1155/ijh/7385050",
"abstract": "<jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>According to recent research, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can cause liver injury, which may be linked to a poorer prognosis. In this retrospective study, we evaluated the incidence of high liver enzyme levels in patients with COVID‐19 and their correlation with the severity and prognosis of clinical outcomes.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>\n A retrospective cohort study was performed from March 2020 to October 2020 at Prince Mohammed Bin Abdulaziz Hospital (PMAH), Riyadh, Saudi Arabia. Demographic information of COVID‐19 patients as well as data on clinical features and laboratory parameters were collected. Pearson′s correlation (\n <jats:italic>r</jats:italic>\n ) test was used to assess the correlation between elevated liver enzymes and COVID‐19 severity. The multivariate logistic binary regression analysis was used to identify the predictors of elevated liver enzyme levels and mortality among patients with COVID‐19.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>\n This cohort included 1033 patients, 73% of whom were male, with a mean age of 49.9 years. Elevated liver enzymes were observed in 52.7% of patients, most commonly with a hepatitis pattern (63.1%). Elevated levels of hemoglobin, creatine kinase–myocardial band, and C‐reactive protein, as well as pneumoniae, the requirement of an intensive care unit, comorbidities, and the use of paracetamol,\n <jats:italic>β</jats:italic>\n ‐lactamase, and steroids were significant predictors of elevated liver enzymes (\n <jats:italic>p</jats:italic>\n < 0.05). Interestingly, Saudi patients (\n <jats:italic>p</jats:italic>\n = 0.019) were found to be a significant protective predictor of elevated liver enzymes. Our findings revealed that elevated liver enzyme levels were significantly correlated with the severity of COVID‐19 (\n <jats:italic>p</jats:italic>\n < 0.05) in terms of qSOFA score. Moreover, older age, diabetes, qSOFA score, and elevated hepatitis enzymes were associated with mortality (\n <jats:italic>p</jats:italic>\n = 0.043).\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Elevated liver enzyme levels were common in patients with COVID‐19 and were associated with the severity and prognosis of clinical outcomes.</jats:p>\n </jats:sec>",
"alternative-id": [
"10.1155/ijh/7385050"
],
"article-number": "7385050",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2025-07-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-10-15"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-11-26"
}
],
"author": [
{
"ORCID": "https://orcid.org/0009-0006-2614-0249",
"affiliation": [],
"authenticated-orcid": false,
"family": "Makkawy",
"given": "Eyad",
"sequence": "first"
},
{
"affiliation": [],
"family": "Alamro",
"given": "Shaden Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibn Awadh",
"given": "Safiya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Basalasil",
"given": "Reem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alkhelaiwi",
"given": "Ziyad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AL-Mutairi",
"given": "Bassam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rebh",
"given": "Fatimah",
"sequence": "additional"
}
],
"container-title": "International Journal of Hepatology",
"container-title-short": "International Journal of Hepatology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2025,
11,
26
]
],
"date-time": "2025-11-26T14:47:38Z",
"timestamp": 1764168458000
},
"deposited": {
"date-parts": [
[
2025,
11,
26
]
],
"date-time": "2025-11-26T14:47:40Z",
"timestamp": 1764168460000
},
"editor": [
{
"affiliation": [],
"family": "Mircea-Catalin",
"given": "Fortofoiu",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2025,
11,
26
]
],
"date-time": "2025-11-26T14:51:18Z",
"timestamp": 1764168678687,
"version": "3.46.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2025,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 329,
"start": {
"date-parts": [
[
2025,
11,
26
]
],
"date-time": "2025-11-26T00:00:00Z",
"timestamp": 1764115200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1155/ijh/7385050",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1155",
"published": {
"date-parts": [
[
2025,
1
]
]
},
"published-online": {
"date-parts": [
[
2025,
11,
26
]
]
},
"published-print": {
"date-parts": [
[
2025,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1111/liv.14470",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_1_2"
},
{
"key": "e_1_2_11_2_2",
"unstructured": "World Health Organization Coronavirus Disease (COVID-19) Pandemic 2020."
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_2"
},
{
"DOI": "10.15698/cst2020.04.216",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_2"
},
{
"DOI": "10.1002/path.1560",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_2"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_2"
},
{
"DOI": "10.1007/s12072-020-10078-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_2"
},
{
"DOI": "10.1016/j.jhepr.2020.100113",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_2"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_2"
},
{
"DOI": "10.4254/wjh.v13.i11.1688",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_2"
},
{
"DOI": "10.1002/hep.31472",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_2"
},
{
"DOI": "10.1111/joim.13292",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_2"
},
{
"DOI": "10.1177/20503121231208648",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_2"
},
{
"DOI": "10.1590/s0004-2803.202301000-03",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_2"
},
{
"DOI": "10.1002/hep.31446",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_2"
},
{
"DOI": "10.1016/S2468-1253(20)30057-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_2"
},
{
"DOI": "10.1136/gutjnl-2020-321726",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_2"
},
{
"DOI": "10.1159/000510758",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_2"
},
{
"DOI": "10.1002/hep.31301",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_2"
},
{
"DOI": "10.1016/j.ijid.2020.04.041",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_2"
},
{
"DOI": "10.1002/hep.21593",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_2"
},
{
"DOI": "10.3390/medicina58121848",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_2"
},
{
"DOI": "10.3748/wjg.v28.i47.6662",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_2"
},
{
"DOI": "10.1016/j.resp.2020.103548",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_2"
},
{
"DOI": "10.1016/j.jhep.2020.04.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_2"
},
{
"DOI": "10.3748/wjg.v26.i48.7693",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_2"
},
{
"DOI": "10.1038/s41575-021-00426-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_2"
},
{
"DOI": "10.1111/liv.14730",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_28_2"
},
{
"DOI": "10.1002/hep.31404",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_2"
},
{
"DOI": "10.1016/j.livres.2021.01.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_2"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1155/ijh/7385050"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Impact of Elevated Liver Enzymes on the Severity of Clinical Course of COVID‐19: A Retrospective Study From Saudi Arabia",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "2025"
}