The effects of urine alkalinization on kidney function in critically ill patients with COVID-19: a proof-of-concept randomized clinical trial
et al., Intensive Care Medicine Experimental, doi:10.1186/s40635-025-00739-7, NCT04655716, Mar 2025
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0000000039 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
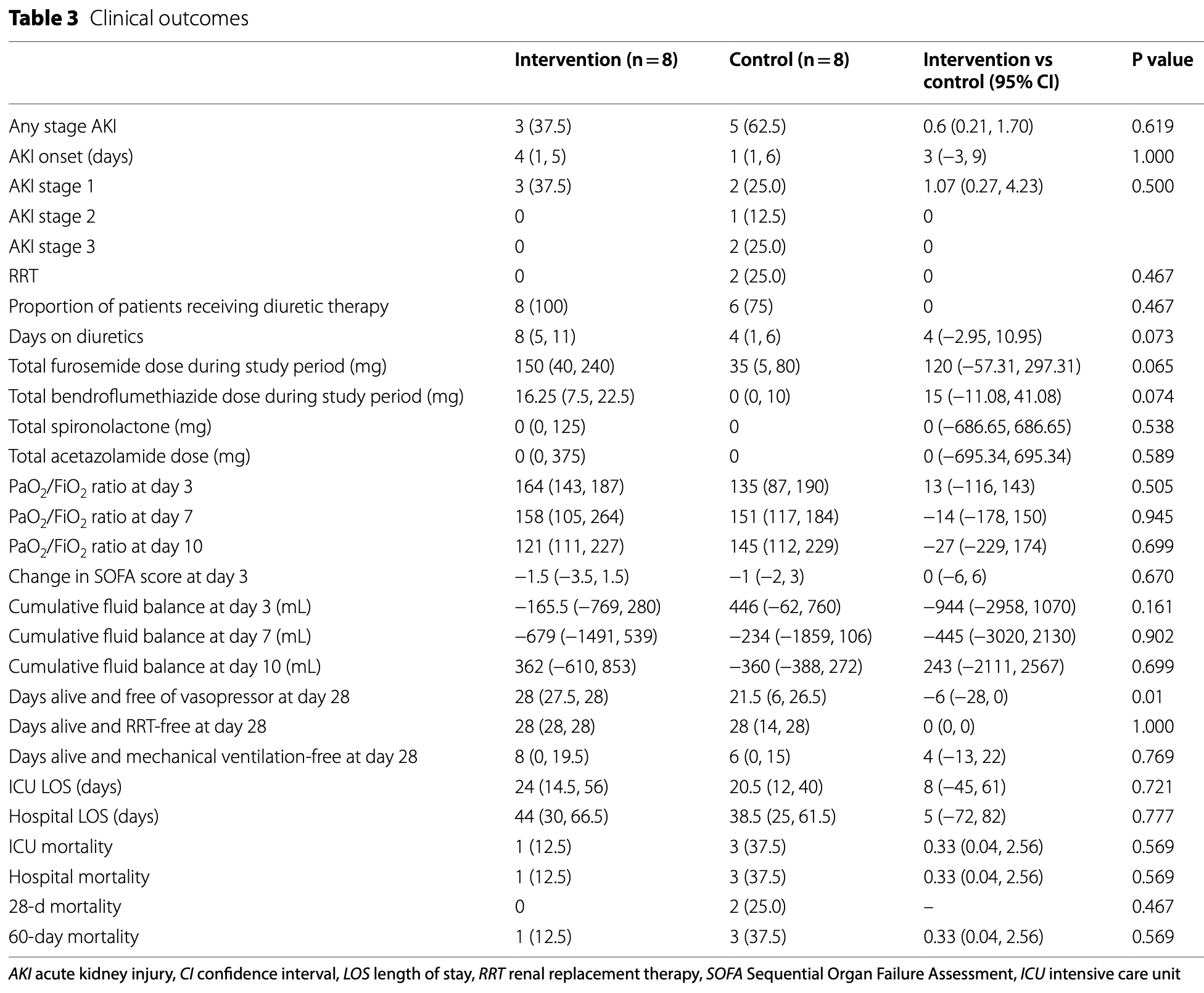
Early terminated RCT 16 critically ill COVID-19 patients showing no significant difference in AKI development or mortality with alkalinization using intravenous sodium bicarbonate. The intervention group achieved higher urine pH (75% vs 37.5% reached pH ≥ 7.5), but AKI incidence (37.5% vs 62.5%) and 60-day mortality (12.5% vs 37.5%) were not statistically different. Recruitment stopped early due to declining COVID-19 ICU admissions, limiting sample size.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 66.7% lower, RR 0.33, p = 0.57, treatment 1 of 8 (12.5%), control 3 of 8 (37.5%), NNT 4.0, day 60.
|
|
AKI, 40.0% lower, RR 0.60, p = 0.62, treatment 3 of 8 (37.5%), control 5 of 8 (62.5%), NNT 4.0.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lumlertgul et al., 7 Mar 2025, Randomized Controlled Trial, United Kingdom, peer-reviewed, median age 48.0, 6 authors, study period July 2021 - January 2022, trial NCT04655716 (history).
Contact: marlies.ostermann@gstt.nhs.uk.
The effects of urine alkalinization on kidney function in critically ill patients with COVID-19: a proof-of-concept randomized clinical trial
Intensive Care Medicine Experimental, doi:10.1186/s40635-025-00739-7
Background Acute kidney injury (AKI) is a common complication of COVID-19. While the exact mechanisms remain unclear, direct viral infection of renal tubular epithelial cells is hypothesized. Given the pH-dependent entry of coronaviruses into host cells, urine alkalinization was proposed as a potential preventive strategy.
Methods This was a proof-of-concept prospective, randomized clinical trial in critically ill patients with COVID-19. Patients were randomized to urine alkalinization versus usual care. The intervention group received intravenous 8.4% sodium bicarbonate to achieve a urine pH ≥ 7.5 up to 10 days after randomization. The primary outcome was the proportion of patients achieving target urine pH. Secondary outcomes included changes in urine tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7), AKI development, renal replacement therapy, and adverse effects.
Results The trial was terminated early due to slow recruitment and the end of the COVID-19 pandemic. Sixteen patients were enrolled (median age 48 years old, 75% male). More patients in the intervention group achieved target urine pH than in the control group (75% vs 37.5%, P = 0.315). There was a separation of urine pH between both groups throughout 10 days (P = 0.097 for interaction). However, the intervention did not significantly impact urine [TIMP-2] x[IGFBP7] concentrations (P = 0.813 for interaction) or clinical outcomes, including AKI occurrence (risk ratio 0.6 (95% confidence interval 0.21, 1.70), P = 0.619). More patients in the intervention group experienced hypernatremia and metabolic alkalosis. Notably, patients with elevated urine [TIMP-2]x[IGFBP7] concentrations and AKI had higher ICU and 60-day mortality. Conclusions While urine alkalinization is feasible and can increase urine pH, we could not demonstrate differences in AKI rates or changes in urine x[IGFBP7] concentrations in critically ill COVID-19 patients.
Abbreviations
ACE2 Angiotensin-converting enzyme-
Supplementary Information The online version contains supplementary material available at https:// doi . org/ 10. 1186/ s40635-025-00739-7. Additional file 1
Author contributions All authors contributed directly to the study conception and design. JAK, MO, and NL developed the study protocol. Data collection and statistical analyses were performed by NL. MM was responsible for management of the investigational medical product. SS performed the laboratory analyses. The first draft of the manuscript was written by NL. All authors commented on versions of the manuscript. All authors read and approved the final manuscript.
Declarations Ethics approval and consent to participate The study was approved by a National Research Ethics Committee in the UK (London-Harrow) (REC reference 21/HRA/0440) and carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from participants or provided by a personal or professional consultee if the patient did not have capacity to consent for themselves. In a case where a consultee had given permission for the patient to take part, the patient was invited to give informed consent to continue participation or to withdraw from further participation as soon as they regained capacity.
Consent for publication Written consent for publication was obtained from participants or provided by a personal or..
References
Braun, Lütgehetmann, Pfefferle, Wong, Carsten et al., SARS-CoV-2 renal tropism associates with acute kidney injury, Lancet
Chu, Mcelroy, Chu, Bauman, Whittaker, The avian coronavirus infectious bronchitis virus undergoes direct low-pH-dependent fusion activation during entry into host cells, J Virol
Cicconetti, Sestili, Madiai, Albertini, Campanella et al., Extracellular pH, osmolarity, temperature and humidity could discourage SARS-CoV-2 cell docking and propagation via intercellular signaling pathways, PeerJ
Clemmons, Chase, Duong, Waller, Saunders et al., Assessing the impact of adding acetazolamide to oral or intravenous sodium bicarbonate as compared with intravenous bicarbonate monotherapy as urinary alkalinization in adults receiving high-dose methotrexate, Support Care Cancer
Hanley, Naresh, Roufosse, Nicholson, Weir et al., Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study, Lancet Microbe
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Jaber, Paugam, Futier, Lefrant, Lasocki et al., Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial, Lancet
Khwaja, KDIGO clinical practice guidelines for acute kidney injury, Nephron Clin Pract
Korucu, Unal, Pekcan, Inkaya, Yeter et al., Ultrastructural evaluation of urine alkalinization versus hydration on colistin-induced nephrotoxicity, Hum Exp Toxicol
Kreutzberger, Sanyal, Saminathan, Bloyet, Stumpf et al., SARS-CoV-2 requires acidic pH to infect cells, Proc Natl Acad Sci U S A
Lumlertgul, Pirondini, Cooney, Kok, Gregson et al., Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study, Ann Intensive Care
Matsumoto, Prowle, COVID-19-associated AKI, Curr Opin Crit Care
Nadim, Forni, Mehta, Connor, Jr et al., COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup, Nat Rev Nephrol
Neto, Fujii, Mcnamara, Moore, Young et al., Sodium bicarbonate for metabolic acidosis in the ICU: results of a pilot randomized double-blind clinical trial, Crit Care Med
Ostermann, Liu, Pathophysiology of AKI, Best Pract Res Clin Anaesthesiol
Ostermann, Lumlertgul, Forni, Hoste, What every Intensivist should know about COVID-19 associated acute kidney injury, J Crit Care
Ostermann, Lumlertgul, Jeong, Joannidis, James, Acute kidney injury, Lancet
Passoni, Lordani, Peres, Carvalho, Occurrence of acute kidney injury in adult patients hospitalized with COVID-19: a systematic review and meta-analysis, Nefrologia (Engl Ed)
Proudfoot, Krenzelok, Vale, Position paper on urine alkalinization, J Toxicol Clin Toxicol
Puelles, Lütgehetmann, Lindenmeyer, Sperhake, Wong et al., Multiorgan and Renal Tropism of SARS-CoV-2, N Engl J Med
Ray, Patel, Irsik, Sun, Ocasio et al., Sodium bicarbonate loading limits tubular cast formation independent of glomerular injury and proteinuria in Dahl saltsensitive rats, Clin Sci (Lond)
Ren, Glende, Al-Falah, De Vries, Schwegmann-Wessels et al., Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus, J Gen Virol
Schurink, Roos, Radonic, Barbe, Bouman et al., Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study, Lancet Microbe
Shafiee, Jafarabady, Moltazemi, Amini, Rafiei et al., Alkalinization using sodium bicarbonate for COVID-19 treatment: a systematic review and meta-analysis, J Evid Based Integr Med, doi:10.1177/2515690X241258403
Souma, Abe, Moriguchi, Takai, Yanagisawa-Miyazawa et al., Luminal alkalinization attenuates proteinuria-induced oxidative damage in proximal tubular cells, J Am Soc Nephrol
Sturman, Ricard, Holmes, Conformational change of the coronavirus peplomer glycoprotein at pH 80 and 37 degrees C correlates with virus aggregation and virus-induced cell fusion, J Virol
Sullivan, Lees, Drake, Docherty, Oates et al., Acute kidney injury in patients hospitalized with COVID-19 from the ISARIC WHO CCP-UK Study: a prospective, multicentre cohort study, Nephrol Dial Transplant
Tai, He, Zhang, Pu, Voronin et al., Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine, Cell Mol Immunol
Weiss, Von Groote, Ostermann, Lumlertgul, Weerapolchai et al., The role of cell cycle arrest biomarkers for predicting acute kidney injury in critically Ill COVID-19 patients: a multicenter, Obs Study Crit Care Med
Zhang, Zhao, Wang, Zheng, Xu et al., Long-term renal outcomes of patients with COVID-19: a meta-analysis of observational studies, J Nephrol
Zhou, Xie, Tang, Pu, Zhu et al., Therapeutic targets and interventional strategies in COVID-19: mechanisms and clinical studies, Sig Transduct Target Ther
DOI record:
{
"DOI": "10.1186/s40635-025-00739-7",
"ISSN": [
"2197-425X"
],
"URL": "http://dx.doi.org/10.1186/s40635-025-00739-7",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Acute kidney injury (AKI) is a common complication of COVID-19. While the exact mechanisms remain unclear, direct viral infection of renal tubular epithelial cells is hypothesized. Given the pH-dependent entry of coronaviruses into host cells, urine alkalinization was proposed as a potential preventive strategy.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This was a proof-of-concept prospective, randomized clinical trial in critically ill patients with COVID-19. Patients were randomized to urine alkalinization versus usual care. The intervention group received intravenous 8.4% sodium bicarbonate to achieve a urine pH ≥ 7.5 up to 10 days after randomization. The primary outcome was the proportion of patients achieving target urine pH. Secondary outcomes included changes in urine tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7), AKI development, renal replacement therapy, and adverse effects.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The trial was terminated early due to slow recruitment and the end of the COVID-19 pandemic. Sixteen patients were enrolled (median age 48 years old, 75% male). More patients in the intervention group achieved target urine pH than in the control group (75% vs 37.5%, <jats:italic>P</jats:italic> = 0.315). There was a separation of urine pH between both groups throughout 10 days (<jats:italic>P</jats:italic> = 0.097 for interaction). However, the intervention did not significantly impact urine [TIMP-2]x[IGFBP7] concentrations (<jats:italic>P</jats:italic> = 0.813 for interaction) or clinical outcomes, including AKI occurrence (risk ratio 0.6 (95% confidence interval 0.21, 1.70), <jats:italic>P</jats:italic> = 0.619). More patients in the intervention group experienced hypernatremia and metabolic alkalosis. Notably, patients with elevated urine [TIMP-2]x[IGFBP7] concentrations and AKI had higher ICU and 60-day mortality.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>While urine alkalinization is feasible and can increase urine pH, we could not demonstrate differences in AKI rates or changes in urine [TIMP-2]x[IGFBP7] concentrations in critically ill COVID-19 patients.</jats:p>\n </jats:sec>",
"alternative-id": [
"739"
],
"article-number": "33",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "16 December 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "23 February 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "7 March 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was approved by a National Research Ethics Committee in the UK (London—Harrow) (REC reference 21/HRA/0440) and carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from participants or provided by a personal or professional consultee if the patient did not have capacity to consent for themselves. In a case where a consultee had given permission for the patient to take part, the patient was invited to give informed consent to continue participation or to withdraw from further participation as soon as they regained capacity."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Written consent for publication was obtained from participants or provided by a personal or professional consultee if the patient did not have capacity to consent for themselves. In a case where a consultee gave permission for the patient to take part, the patient was informed and invited to give informed consent for publication as soon as they regained capacity."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "MO has received research funding from Baxter and Biomerieux. The funding was paid to the institution. JAK discloses consulting fees from BioMérieux, AstraZeneca, Bayer, Novartis, Mitsubishi Tenabe, and Chugai Pharma; and employment and stock with Spectral Medical. NL, JPT, MM, and SS declare no conflict of interest."
}
],
"author": [
{
"affiliation": [],
"family": "Lumlertgul",
"given": "Nuttha",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kellum",
"given": "John A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Powell-Tuck",
"given": "Jonah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mathew",
"given": "Moncy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sardiwal",
"given": "Sunita",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9500-9080",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ostermann",
"given": "Marlies",
"sequence": "additional"
}
],
"container-title": "Intensive Care Medicine Experimental",
"container-title-short": "ICMx",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
3,
7
]
],
"date-time": "2025-03-07T16:33:31Z",
"timestamp": 1741365211000
},
"deposited": {
"date-parts": [
[
2025,
3,
7
]
],
"date-time": "2025-03-07T16:33:35Z",
"timestamp": 1741365215000
},
"funder": [
{
"name": "Baxter Global"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
7
]
],
"date-time": "2025-03-07T17:10:20Z",
"timestamp": 1741367420314,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
3,
7
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
7
]
],
"date-time": "2025-03-07T00:00:00Z",
"timestamp": 1741305600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
7
]
],
"date-time": "2025-03-07T00:00:00Z",
"timestamp": 1741305600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40635-025-00739-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40635-025-00739-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40635-025-00739-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
3,
7
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
7
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1093/ndt/gfab303",
"author": "MK Sullivan",
"doi-asserted-by": "publisher",
"first-page": "271",
"issue": "2",
"journal-title": "Nephrol Dial Transplant",
"key": "739_CR1",
"unstructured": "Sullivan MK, Lees JS, Drake TM, Docherty AB, Oates G, Hardwick HE, Russell CD, Merson L, Dunning J, Nguyen-Van-Tam JS et al (2022) Acute kidney injury in patients hospitalized with COVID-19 from the ISARIC WHO CCP-UK Study: a prospective, multicentre cohort study. Nephrol Dial Transplant 37(2):271–284",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1097/MCC.0000000000000988",
"author": "K Matsumoto",
"doi-asserted-by": "publisher",
"first-page": "630",
"issue": "6",
"journal-title": "Curr Opin Crit Care",
"key": "739_CR2",
"unstructured": "Matsumoto K, Prowle JR (2022) COVID-19-associated AKI. Curr Opin Crit Care 28(6):630–637",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1186/s13613-021-00914-5",
"author": "N Lumlertgul",
"doi-asserted-by": "publisher",
"first-page": "123",
"issue": "1",
"journal-title": "Ann Intensive Care",
"key": "739_CR3",
"unstructured": "Lumlertgul N, Pirondini L, Cooney E, Kok W, Gregson J, Camporota L, Lane K, Leach R, Ostermann M (2021) Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care 11(1):123",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.nefroe.2022.11.005",
"author": "R Passoni",
"doi-asserted-by": "publisher",
"first-page": "404",
"issue": "4",
"journal-title": "Nefrologia (Engl Ed)",
"key": "739_CR4",
"unstructured": "Passoni R, Lordani TVA, Peres LAB, Carvalho A (2022) Occurrence of acute kidney injury in adult patients hospitalized with COVID-19: a systematic review and meta-analysis. Nefrologia (Engl Ed) 42(4):404–414",
"volume": "42",
"year": "2022"
},
{
"DOI": "10.1007/s40620-023-01731-8",
"author": "Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "2441",
"issue": "9",
"journal-title": "J Nephrol",
"key": "739_CR5",
"unstructured": "Zhang Y, Zhao Y, Wang J, Zheng X, Xu D, Lv J, Yang L (2023) Long-term renal outcomes of patients with COVID-19: a meta-analysis of observational studies. J Nephrol 36(9):2441–2456",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.1038/s41581-020-00356-5",
"author": "MK Nadim",
"doi-asserted-by": "publisher",
"first-page": "747",
"issue": "12",
"journal-title": "Nat Rev Nephrol",
"key": "739_CR6",
"unstructured": "Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, Rimmelé T, Zarbock A, Bell S, Bihorac A et al (2020) COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol 16(12):747–764",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2020.07.023",
"author": "M Ostermann",
"doi-asserted-by": "publisher",
"first-page": "91",
"journal-title": "J Crit Care",
"key": "739_CR7",
"unstructured": "Ostermann M, Lumlertgul N, Forni LG, Hoste E (2020) What every Intensivist should know about COVID-19 associated acute kidney injury. J Crit Care 60:91–95",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1016/j.bpa.2017.09.001",
"author": "M Ostermann",
"doi-asserted-by": "publisher",
"first-page": "305",
"issue": "3",
"journal-title": "Best Pract Res Clin Anaesthesiol",
"key": "739_CR8",
"unstructured": "Ostermann M, Liu K (2017) Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol 31(3):305–314",
"volume": "31",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(24)02385-7",
"author": "M Ostermann",
"doi-asserted-by": "publisher",
"first-page": "241",
"issue": "10474",
"journal-title": "Lancet",
"key": "739_CR9",
"unstructured": "Ostermann M, Lumlertgul N, Jeong R, See E, Joannidis M, James M (2025) Acute kidney injury. Lancet 405(10474):241–256",
"volume": "405",
"year": "2025"
},
{
"DOI": "10.1038/s41423-020-0400-4",
"author": "W Tai",
"doi-asserted-by": "publisher",
"first-page": "613",
"issue": "6",
"journal-title": "Cell Mol Immunol",
"key": "739_CR10",
"unstructured": "Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L (2020) Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 17(6):613–620",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1099/vir.0.81749-0",
"author": "X Ren",
"doi-asserted-by": "publisher",
"first-page": "1691",
"issue": "Pt 6",
"journal-title": "J Gen Virol",
"key": "739_CR11",
"unstructured": "Ren X, Glende J, Al-Falah M, de Vries V, Schwegmann-Wessels C, Qu X, Tan L, Tschernig T, Deng H, Naim HY et al (2006) Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J Gen Virol 87(Pt 6):1691–1695",
"volume": "87",
"year": "2006"
},
{
"DOI": "10.1016/S2666-5247(20)30144-0",
"author": "B Schurink",
"doi-asserted-by": "publisher",
"first-page": "e290",
"issue": "7",
"journal-title": "Lancet Microbe",
"key": "739_CR12",
"unstructured": "Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S et al (2020) Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 1(7):e290–e299",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(20)30115-4",
"author": "B Hanley",
"doi-asserted-by": "publisher",
"first-page": "e245",
"issue": "6",
"journal-title": "Lancet Microbe",
"key": "739_CR13",
"unstructured": "Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R et al (2020) Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 1(6):e245–e253",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31759-1",
"author": "F Braun",
"doi-asserted-by": "publisher",
"first-page": "597",
"issue": "10251",
"journal-title": "Lancet",
"key": "739_CR14",
"unstructured": "Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D et al (2020) SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396(10251):597–598",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2011400",
"author": "VG Puelles",
"doi-asserted-by": "publisher",
"first-page": "590",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "739_CR15",
"unstructured": "Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S et al (2020) Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med 383(6):590–592",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "739_CR16",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e278",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2209514119",
"author": "AJB Kreutzberger",
"doi-asserted-by": "publisher",
"issue": "38",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "739_CR17",
"unstructured": "Kreutzberger AJB, Sanyal A, Saminathan A, Bloyet LM, Stumpf S, Liu Z, Ojha R, Patjas MT, Geneid A, Scanavachi G et al (2022) SARS-CoV-2 requires acidic pH to infect cells. Proc Natl Acad Sci U S A 119(38):e2209514119",
"volume": "119",
"year": "2022"
},
{
"DOI": "10.7717/peerj.12227",
"author": "F Cicconetti",
"doi-asserted-by": "publisher",
"journal-title": "PeerJ",
"key": "739_CR18",
"unstructured": "Cicconetti F, Sestili P, Madiai V, Albertini MC, Campanella L, Coppari S, Fraternale D, Saunders B, Teodori L (2021) Extracellular pH, osmolarity, temperature and humidity could discourage SARS-CoV-2 cell docking and propagation via intercellular signaling pathways. PeerJ 9:e12227",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1128/jvi.64.6.3042-3050.1990",
"author": "LS Sturman",
"doi-asserted-by": "publisher",
"first-page": "3042",
"issue": "6",
"journal-title": "J Virol",
"key": "739_CR19",
"unstructured": "Sturman LS, Ricard CS, Holmes KV (1990) Conformational change of the coronavirus peplomer glycoprotein at pH 80 and 37 degrees C correlates with virus aggregation and virus-induced cell fusion. J Virol 64(6):3042–3050",
"volume": "64",
"year": "1990"
},
{
"DOI": "10.1038/s41392-021-00733-x",
"author": "Y-W Zhou",
"doi-asserted-by": "publisher",
"first-page": "317",
"issue": "1",
"journal-title": "Sig Transduct Target Ther",
"key": "739_CR20",
"unstructured": "Zhou Y-W, Xie Y, Tang L-S, Pu D, Zhu Y-J, Liu J-Y, Ma X-L (2021) Therapeutic targets and interventional strategies in COVID-19: mechanisms and clinical studies. Sig Transduct Target Ther 6(1):317",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1081/CLT-120028740",
"author": "AT Proudfoot",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "J Toxicol Clin Toxicol",
"key": "739_CR21",
"unstructured": "Proudfoot AT, Krenzelok EP, Vale JA (2004) Position paper on urine alkalinization. J Toxicol Clin Toxicol 42(1):1–26",
"volume": "42",
"year": "2004"
},
{
"DOI": "10.1159/000339789",
"author": "A Khwaja",
"doi-asserted-by": "publisher",
"first-page": "c179",
"issue": "4",
"journal-title": "Nephron Clin Pract",
"key": "739_CR22",
"unstructured": "Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179-184",
"volume": "120",
"year": "2012"
},
{
"DOI": "10.1128/JVI.80.7.3180-3188.2006",
"author": "VC Chu",
"doi-asserted-by": "publisher",
"first-page": "3180",
"issue": "7",
"journal-title": "J Virol",
"key": "739_CR23",
"unstructured": "Chu VC, McElroy LJ, Chu V, Bauman BE, Whittaker GR (2006) The avian coronavirus infectious bronchitis virus undergoes direct low-pH-dependent fusion activation during entry into host cells. J Virol 80(7):3180–3188",
"volume": "80",
"year": "2006"
},
{
"DOI": "10.1177/2515690X241258403",
"author": "A Shafiee",
"doi-asserted-by": "publisher",
"journal-title": "J Evid Based Integr Med",
"key": "739_CR24",
"unstructured": "Shafiee A, Jafarabady K, Moltazemi H, Amini MJ, Rafiei MA, Akhondi A, Mozhgani SH (2024) Alkalinization using sodium bicarbonate for COVID-19 treatment: a systematic review and meta-analysis. J Evid Based Integr Med. https://doi.org/10.1177/2515690X241258403",
"year": "2024"
},
{
"DOI": "10.1007/s00520-020-05646-z",
"author": "AB Clemmons",
"doi-asserted-by": "publisher",
"first-page": "1527",
"issue": "3",
"journal-title": "Support Care Cancer",
"key": "739_CR25",
"unstructured": "Clemmons AB, Chase A, Duong P, Waller JL, Saunders K, Bryan L (2021) Assessing the impact of adding acetazolamide to oral or intravenous sodium bicarbonate as compared with intravenous bicarbonate monotherapy as urinary alkalinization in adults receiving high-dose methotrexate. Support Care Cancer 29(3):1527–1534",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000005955",
"author": "A Serpa Neto",
"doi-asserted-by": "publisher",
"first-page": "e221",
"issue": "11",
"journal-title": "Crit Care Med",
"key": "739_CR26",
"unstructured": "Serpa Neto A, Fujii T, McNamara M, Moore J, Young PJ, Peake S, Bailey M, Hodgson C, Higgins AM, See EJ et al (2023) Sodium bicarbonate for metabolic acidosis in the ICU: results of a pilot randomized double-blind clinical trial. Crit Care Med 51(11):e221–e233",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(18)31080-8",
"author": "S Jaber",
"doi-asserted-by": "publisher",
"first-page": "31",
"issue": "10141",
"journal-title": "Lancet",
"key": "739_CR27",
"unstructured": "Jaber S, Paugam C, Futier E, Lefrant JY, Lasocki S, Lescot T, Pottecher J, Demoule A, Ferrandière M, Asehnoune K et al (2018) Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet 392(10141):31–40",
"volume": "392",
"year": "2018"
},
{
"DOI": "10.1177/0960327119862339",
"author": "B Korucu",
"doi-asserted-by": "publisher",
"first-page": "1366",
"issue": "12",
"journal-title": "Hum Exp Toxicol",
"key": "739_CR28",
"unstructured": "Korucu B, Unal I, Pekcan M, Inkaya AC, Yeter H, Cetinkaya MA, Kaymaz FF, Unal S, Akova M, Erdem Y (2019) Ultrastructural evaluation of urine alkalinization versus hydration on colistin-induced nephrotoxicity. Hum Exp Toxicol 38(12):1366–1377",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.1042/CS20171630",
"author": "SC Ray",
"doi-asserted-by": "publisher",
"first-page": "1179",
"issue": "11",
"journal-title": "Clin Sci (Lond)",
"key": "739_CR29",
"unstructured": "Ray SC, Patel B, Irsik DL, Sun J, Ocasio H, Crislip GR, Jin CH, Chen J, Baban B, Polichnowski AJ et al (2018) Sodium bicarbonate loading limits tubular cast formation independent of glomerular injury and proteinuria in Dahl salt-sensitive rats. Clin Sci (Lond) 132(11):1179–1197",
"volume": "132",
"year": "2018"
},
{
"DOI": "10.1681/ASN.2009111130",
"author": "T Souma",
"doi-asserted-by": "publisher",
"first-page": "635",
"issue": "4",
"journal-title": "J Am Soc Nephrol",
"key": "739_CR30",
"unstructured": "Souma T, Abe M, Moriguchi T, Takai J, Yanagisawa-Miyazawa N, Shibata E, Akiyama Y, Toyohara T, Suzuki T, Tanemoto M et al (2011) Luminal alkalinization attenuates proteinuria-induced oxidative damage in proximal tubular cells. J Am Soc Nephrol 22(4):635–648",
"volume": "22",
"year": "2011"
},
{
"DOI": "10.1097/CCM.0000000000005853",
"author": "R Weiss",
"doi-asserted-by": "publisher",
"first-page": "992",
"issue": "8",
"journal-title": "Obs Study Crit Care Med",
"key": "739_CR31",
"unstructured": "Weiss R, von Groote T, Ostermann M, Lumlertgul N, Weerapolchai K, Garcia MIM, Cano JMM, Del Corral BD, Broch-Porcar MJ, Perez Carrasco M et al (2023) The role of cell cycle arrest biomarkers for predicting acute kidney injury in critically Ill COVID-19 patients: a multicenter. Obs Study Crit Care Med 51(8):992–1000",
"volume": "51",
"year": "2023"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://icm-experimental.springeropen.com/articles/10.1186/s40635-025-00739-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The effects of urine alkalinization on kidney function in critically ill patients with COVID-19: a proof-of-concept randomized clinical trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}
