Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: retrospective observational study
et al., BMJ, doi:10.1136/bmj.j4619, Oct 2017
Analysis of industry payments to US medical journal editors in 2014 using the Open Payments database. Payments were common and often large. Only about a third of journals had readily accessible editorial conflict of interest policies. Authors suggest journals should adopt more transparent editorial conflict of interest policies and consider excluding editors with significant industry ties.
Liu et al., 26 Oct 2017, Canada, peer-reviewed, 5 authors.
Contact: jessica.liu@uhn.ca.
Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: retrospective observational study
BMJ, doi:10.1136/bmj.j4619
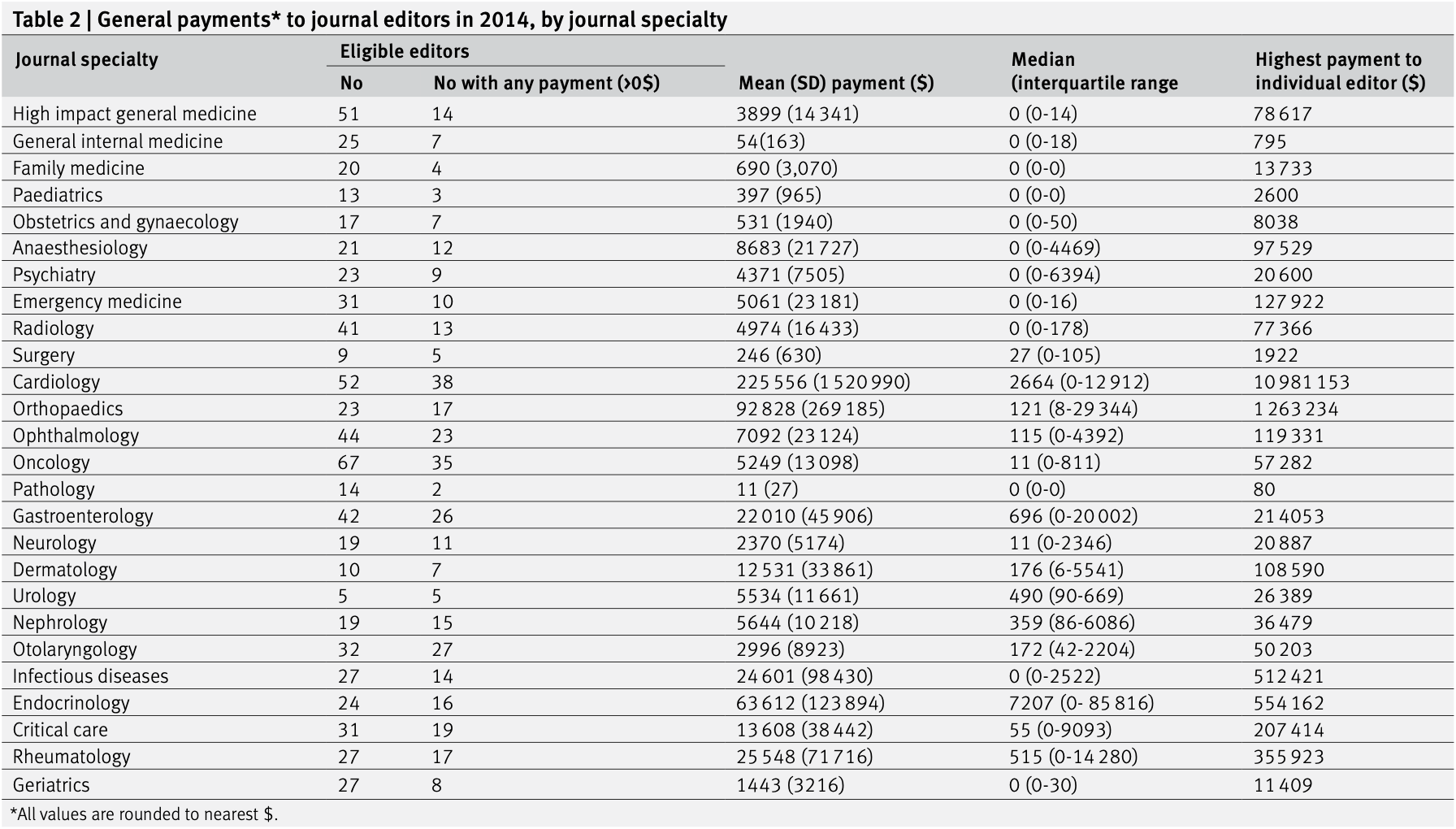
factor for their specialty) US medical journals from 26 specialties and US Open Payments database, 2014. PARTICIPANTS 713 editors at the associate level and above identified from each journal's online masthead.
MAIN OUTCOME MEASURES All general payments (eg, personal income) and research related payments from pharmaceutical and medical device manufacturers to eligible physicians in 2014. Percentages of editors receiving payments and the magnitude of such payments were compared across journals and by specialty. Journal websites were also reviewed to determine if conflict of interest policies for editors were readily accessible.
RESULTS Of 713 eligible editors, 361 (50.6%) received some (>$0) general payments in 2014, and 139 (19.5%) received research payments. The median general payment was $11 (£8; €9) (interquartile range $0-2923) and the median research payment was $0 ($0-0). The mean general payment was $28 136 (SD $415 045), and the mean research payment was $37 963 (SD $175 239). The highest median general payments were received by journal editors from endocrinology ($7207, $0-85 816), cardiology ($2664, $0-12 912), gastroenterology ($696, $0-20 002), rheumatology ($515, $0-14 280), and urology ($480, $90-669). For high impact general medicine journals, median payments were $0 ($0-14). A review of the 52 journal websites revealed that editor conflict of interest policies were readily accessible (ie, within five minutes) for 17/52 (32.7%) of journals.
CONCLUSIONS Industry payments to journal editors are common and often large, particularly for certain subspecialties.
References
Agrawal, Brennan, Budetti, The Sunshine Act--effects on physicians, N Engl J Med, doi:10.1056/NEJMp1303523
Anderson, Good, Gellad, Prevalence and compensation of academic leaders, professors, and trustees on publicly traded US healthcare company boards of directors: cross sectional study, BMJ, doi:10.1136/bmj.h4826
Andreatos, Zacharioudakis, Zervou, Muhammed, Mylonakis, Discrepancy between financial disclosures of authors of clinical practice guidelines and reports by industry, Medicine, doi:10.1097/MD.0000000000005711
Benson, Eyes wide open: reader and author responsibility in understanding the limits of peer review, Ann R Coll Surg Engl, doi:10.1308/rcsann.2015.0032
Blum, Freeman, Dart, Cooper, Requirements and definitions in conflict of interest policies of medical journals, JAMA, doi:10.1001/jama.2009.1669
Bosch, Pericas, Hernández, Doti, Financial, nonfinancial and editors' conflicts of interest in high-impact biomedical journals, Eur J Clin Invest, doi:10.1111/eci.12090
Callaway, Open peer review finds more takers, Nature, doi:10.1038/nature.2016.20969
Campbell, Public disclosure of conflicts of interest: moving the policy debate forward, Arch Intern Med, doi:10.1001/archinternmed.2010.27
Campbell, Weissman, Ehringhaus, Institutional academic industry relationships, JAMA, doi:10.1001/jama.298.15.1779
Campbell, Weissman, Ehringhaus, Institutional academic industry relationships, JAMA, doi:10.1001/jama.298.15.1779
Cooper, Gupta, Wilkes, Hoffman, Conflict of Interest Disclosure Policies and Practices in Peer-reviewed Biomedical Journals, J Gen Intern Med, doi:10.1111/j.1525-1497.2006.00598.x
Davis, Müllner, Editorial independence at medical journals owned by professional associations: a survey of editors, Sci Eng Ethics, doi:10.1007/s11948-002-0004-7
Donovan, Kaplan, Navigating conflicts of interest for the medical device entrepreneur, Prog Cardiovasc Dis
Drazen, De Leeuw, Laine, Toward more uniform conflict disclosures--the updated ICMJE conflict of interest reporting form, N Engl J Med, doi:10.1056/NEJMe1006030
Fleischman, Agrawal, King, Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study, BMJ, doi:10.1136/bmj.i4189
Fontanarosa, Flanagin, Deangelis, Implementation of the ICMJE form for reporting potential conflicts of interest, JAMA, doi:10.1001/jama.2010.1429
Haivas, Schroter, Waechter, Smith, Editors' declaration of their own conflicts of interest, CMAJ, doi:10.1503/cmaj.1031982
Hannon, Chalmers, Carpiniello, Cvetanovich, Cole et al., Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the Open Payments Database[Review], J Bone Joint Surg Am, doi:10.2106/JBJS.15.01119
Hill, Ross, Egilman, Krumholz, The ADVANTAGE seeding trial: a review of internal documents, Ann Intern Med, doi:10.7326/0003-4819-149-4-200808190-00006
Hockenberry, Weigel, Auerbach, Cram, Financial payments by orthopedic device makers to orthopedic surgeons, Arch Intern Med, doi:10.1001/archinternmed.2011.454
Iyer, Derman, Sandhu, Orthopaedics and the Physician Payments Sunshine Act: An Examination of Payments to U.S. Orthopaedic Surgeons in the Open Payments Database, J Bone Joint Surg Am
Janssen, Bredenoord, Dhert, De Kleuver, Oner et al., Potential conflicts of interest of editorial board members from five leading spine journals, PLoS One
Kirschner, Sulmasy, Kesselheim, Health policy basics: the Physician Payment Sunshine Act and the Open Payments program, Ann Intern Med, doi:10.7326/M14-1303
Krumholz, Egilman, Ross, Study of neurontin: titrate to effect, profile of safety (STEPS) trial: a narrative account of a gabapentin seeding trial, Arch Intern Med, doi:10.1001/archinternmed.2011.241
Luty, Arokiadass, Easow, Anapreddy, Preferential publication of editorial board members in medical specialty journals, J Med Ethics, doi:10.1136/jme.2008.026740
Mani, Makarević, Juengel, I publish in I edit?--Do editorial board members of urologic journals preferentially publish their own scientific work?, PLoS One, doi:10.1371/journal.pone.0083709
Marshall, Jackson, Hattangadi-Gluth, Disclosure of Industry Payments to Physicians: An Epidemiologic Analysis of Early Data From the Open Payments Program, Mayo Clin Proc, doi:10.1016/j.mayocp.2015.10.016
Mehlman, Okike, Bhandari, Kocher, Potential financial conflict of interest among physician editorial board members of orthopaedic surgery, J Bone Joint Surg Am, doi:10.2106/JBJS.16.00227
Neuman, Korenstein, Ross, Keyhani, Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study, BMJ, doi:10.1136/bmj.d5621
Norris, Holmer, Ogden, Burda, Fu, Conflicts of interest among authors of clinical practice guidelines for glycemic control in type 2 diabetes mellitus, PLoS One, doi:10.1371/journal.pone.0075284
Okike, Kocher, Wei, Mehlman, Bhandari, Accuracy of conflict-of-interest disclosures reported by physicians, N Engl J Med, doi:10.1056/NEJMsa0807160
Overgaard, Van Den Broek, Kim, Detsky, Biotechnology stock prices before public announcements: evidence of insider trading?, J Investig Med
Parreco, Donath, Kozol, Faber, Comparing industry compensation of cardiothoracic surgeons and interventional cardiologists, J Surg Res
Pisano, Golden, Schweitzer, Conflict of interest policies for academic health system leaders who work with outside corporations, JAMA, doi:10.1001/jama.2014.788
Ray, Judging the judges: the role of journal editors, QJM, doi:10.1093/qjmed/95.12.769
Roseman, Milette, Bero, Reporting of conflicts of interest in meta-analyses of trials of pharmacological treatments, JAMA, doi:10.1001/jama.2011.257
Rosenthal, Mello, Sunlight as disinfectant--new rules on disclosure of industry payments to physicians, N Engl J Med, doi:10.1056/NEJMp1305090312014
Ross, Hill, Egilman, Krumholz, Guest authorship and ghostwriting in publications related to rofecoxib: a case study of industry documents from rofecoxib litigation, JAMA, doi:10.1001/jama.299.15.1800
Rothenstein, Tomlinson, Tannock, Detsky, Company stock prices before and after public announcements related to oncology drugs, J Natl Cancer Inst, doi:10.1093/jnci/djr338
Samuel, Webb, Lukasiewicz, Orthopaedic Surgeons Receive the Most Industry Payments to Physicians but Large Disparities are Seen in Sunshine Act Data, Clin Orthop Relat Res, doi:10.1007/s11999-015-4413-8
Santhakumar, Adashi, The Physician Payment Sunshine Act: testing the value of transparency, JAMA, doi:10.1001/jama.2014.15472
Sklar, What can I believe? Peer review, innovation, and 90 years of academic medicine, Acad Med, doi:10.1097/ACM.0000000000000789
Smith, Medical journals are an extension of the marketing arm of pharmaceutical companies, PLoS Med, doi:10.1371/journal.pmed.0020138
Smith, Opening, BMJ peer review. A beginning that should lead to complete transparency, doi:10.1136/bmj.318.7175.4
Smith, Potvin, Jones, Accessibility and transparency of editor conflicts of interest policy instruments in medical journals, J Med Ethics, doi:10.1136/medethics-2012-100524
Stelfox, Chua, 'rourke, Detsky, Conflict of interest in the debate over calcium-channel antagonists, N Engl J Med, doi:10.1056/NEJM199801083380206
Sullivan, What to Do When Your Paper Is Rejected, J Grad Med Educ, doi:10.4300/JGME-D-14-00686
Thomas, Interventional cardiology and the medical devices industry: is there a conflict of interest?, Heart, doi:10.1136/hrt.2007.123133
Thompson, Volpe, Bridgewater, Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting, Am J Obstet Gynecol, doi:10.1016/j.ajog.2016.06.015
Tringale, Marshall, Mackey, Connor, Murphy et al., Types and Distribution of Payments From Industry to Physicians in 2015, JAMA, doi:10.1001/jama.2017.3091
Van Dijk, Manor, Carey, Publication metrics and success on the academic job market, Curr Biol, doi:10.1016/j.cub.2014.04.039
DOI record:
{
"DOI": "10.1136/bmj.j4619",
"ISSN": [
"0959-8138",
"1756-1833"
],
"URL": "http://dx.doi.org/10.1136/bmj.j4619",
"alternative-id": [
"10.1136/bmj.j4619"
],
"author": [
{
"affiliation": [],
"family": "Liu",
"given": "Jessica J",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bell",
"given": "Chaim M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matelski",
"given": "John J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Detsky",
"given": "Allan S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cram",
"given": "Peter",
"sequence": "additional"
}
],
"container-title": "BMJ",
"container-title-short": "BMJ",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2017,
10,
26
]
],
"date-time": "2017-10-26T14:15:27Z",
"timestamp": 1509027327000
},
"deposited": {
"date-parts": [
[
2020,
9,
26
]
],
"date-time": "2020-09-26T01:28:29Z",
"timestamp": 1601083709000
},
"indexed": {
"date-parts": [
[
2024,
8,
22
]
],
"date-time": "2024-08-22T20:51:52Z",
"timestamp": 1724359912433
},
"is-referenced-by-count": 73,
"issued": {
"date-parts": [
[
2017,
10,
26
]
]
},
"language": "en",
"license": [
{
"URL": "http://www.bmj.org/licenses/tdm/1.0/terms-and-conditions.html",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2017,
10,
26
]
],
"date-time": "2017-10-26T00:00:00Z",
"timestamp": 1508976000000
}
}
],
"link": [
{
"URL": "http://data.bmj.org/tdm/10.1136/bmj.j4619",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmj.j4619",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "j4619",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2017,
10,
26
]
]
},
"published-online": {
"date-parts": [
[
2017,
10,
26
]
]
},
"publisher": "BMJ",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.bmj.com/lookup/doi/10.1136/bmj.j4619"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: retrospective observational study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy"
}