Frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19 in older community-dwelling adults
et al., Aging Clinical and Experimental Research, doi:10.1007/s40520-021-01991-z, Oct 2021
Exercise for COVID-19
9th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 68 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
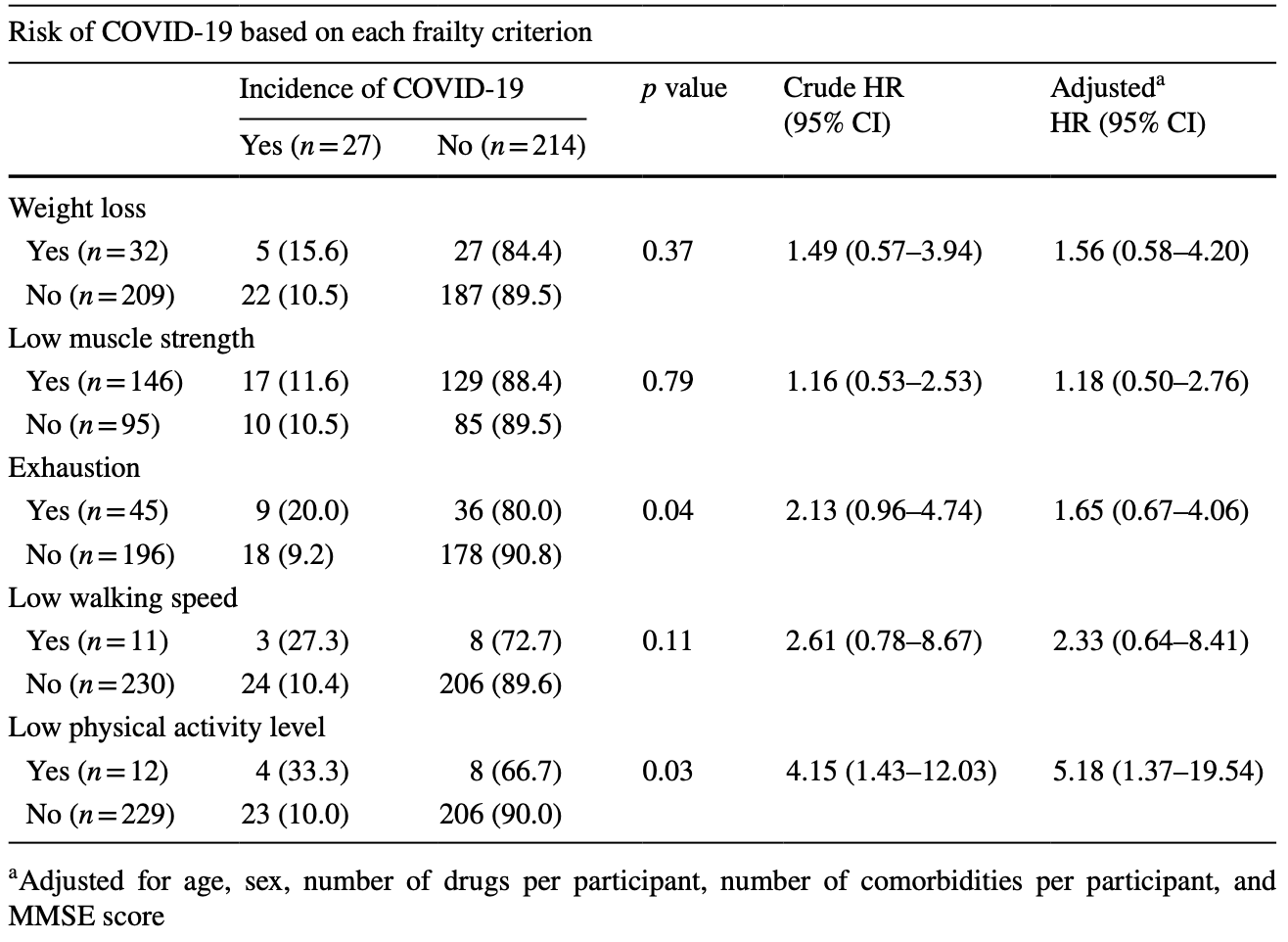
Analysis of 241 adults >65yo in Belgium, showing lower risk of COVID-19 with a history of physical activity.
|
risk of case, 73.6% lower, RR 0.26, p = 0.03, high activity levels 23 of 229 (10.0%), low activity levels 4 of 12 (33.3%), NNT 4.3, inverted to make RR<1 favor high activity levels, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lengelé et al., 23 Oct 2021, prospective, Belgium, peer-reviewed, median age 75.6, 8 authors, study period March 2020 - April 2021.
Contact: llengele@uliege.be.
Frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19 in older community-dwelling adults
Aging Clinical and Experimental Research, doi:10.1007/s40520-021-01991-z
Background The identification of coronavirus disease 2019 (COVID-19) risk factors is requested to implement prevention strategies. Aim To explore the associations between the COVID-19 incidence and malnutrition, sarcopenia, and frailty, identified as potential risk factors in previous cross-sectional studies. Methods Malnutrition, sarcopenia, and frailty were assessed at the last available follow-up from the Sarcopenia and Physical Impairments with Advancing Age (SarcoPhAge) cohort (i.e., the fifth year that ended in 2019) according to the Mini-Nutritional Assessment short-form, the European Working Group on Sarcopenia in Older People (EWGSOP2), and the Fried criteria, respectively. Information regarding the COVID-19 was gathered by phone calls interviews in April 2021 to measure its self-declared incidence. Adjusted Cox regressions and Kaplan-Meier curves were performed.
Results The present study included 241 participants [median age 75.6 (73.0-80.6) years, 63.1% women]. Among them, 27 participants (11.2%) developed the non-fatal Covid-19. No significant increased risks of COVID-19 were observed in patients with malnutrition [adjusted HR 1.14 (0.26-5.07)] and sarcopenia ]. Nevertheless, the incidence of COVID-19 was significantly higher in frail (44.4%) than in robust participants (8.5%) [Adjusted HR 7.01 (2.69-18.25)], which was confirmed by the Kaplan-Meier curves (p < 0.001). Among the frailty syndrome components, a low physical activity level was the only one significantly associated with an increased risk of ]. Conclusion Despite some limitations in the methodology of this study (i.e., limited sample size, COVID-19 incidence selfreported and not assessed systematically using objective measurements) requiring careful consideration, an increased risk to develop COVID-19 was observed in the presence of the frailty syndrome. Further investigations are needed to elaborate on our findings.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s40520-021-01991-z. Author contributions Conceptualization: ML, CB, J-YR, and OB; methodology: LL, ML, MM, CB, J-FK, and OB; formal analysis: LL; investigation: LL, and ML; writing-original draft preparation: LL;
Declarations
Conflict of interest The authors declare no conflict of interest.
Ethical approval The guidelines of the Declaration of Helsinki were followed, and the present study was approved by the ethics committee of the University of Liege Teaching Hospital (reference 2012/277), with two amendments in 2015 and 2018. Human and animal rights All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Consent to participate Informed consent was obtained from all participants involved in the study. Consent for publication Informed consent was obtained from all participants involved in the study. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abate, Chekole, Estifanos, Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: a systematic review and meta-analysis, Clin Nutr ESPEN, doi:10.1016/j.clnesp.2021.03.002
Antonelli, Penfold, Merino, Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00460-6
Bahat, Bauer, Sarcopenia: revised European consensus on definition and diagnosis, Age Ageing, doi:10.1093/ageing/afy169
Bajaj, Gadi, Spihlman, Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections?, Front Physiol, doi:10.3389/fphys.2020.571416
Balestroni, Bertolotti, EuroQol-5D (EQ-5D): an instrument for measuring quality of life, Monaldi Arch Chest Dis, doi:10.4081/monaldi.2012.121
Barazzoni, Bischoff, Breda, ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection, Clin Nutr, doi:10.1016/j.clnu.2020.03.022
Beaudart, Reginster, Petermans, Quality of life and physical components linked to sarcopenia: the Sar-coPhAge study, Exp Gerontol, doi:10.1016/j.exger.2015.05.003
Bencivenga, Rengo, Varricchi, Elderly at time of COronaVIrus disease 2019 (COVID-19): possible role of immunosenescence and malnutrition, Geroscience, doi:10.1007/s11357-020-00218-9
Bokhorst-De, Van Der Schueren, Lonterman-Monasch, Prevalence and determinants for malnutrition in geriatric outpatients, Clin Nutr, doi:10.1016/j.clnu.2013.05.007
Brutto, Costa, Recalde, Frailty and SARS-CoV-2 infection. A population-based study in a highly endemic village, J Neurol Sci, doi:10.1016/j.jns.2020.117136
Cederholm, Barazzoni, Austin, ESPEN guidelines on definitions and terminology of clinical nutrition, Clin Nutr, doi:10.1016/j.clnu.2016.09.004
Cederholm, Overlaps between frailty and sarcopenia definitions, Nestle Nutr Inst Workshop Ser, doi:10.1159/000382063
Chen, Older adults and COVID-19 pandemic: resilience matters, Arch Gerontol Geriatr, doi:10.1016/j.archger.2020.104124
Clegg, Young, Iliffe, Frailty in elderly people, Lancet, doi:10.1016/S0140-6736(12)62167-9
Coelho, Paúl, Gobbens, Determinants of frailty: The added value of assessing medication, Front Aging Neurosci, doi:10.3389/fnagi.2015.00056
Corcoran, Murphy, Culligan, Malnutrition in the elderly, Sci Prog, doi:10.1177/0036850419854290
Da Silveira, Da, Fagundes, Bizuti, Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature, Clin Exp Med, doi:10.1007/s10238-020-00650-3
De, Morais, Aquino, Silva-Maia, Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2, Br J Nutr, doi:10.1017/S0007114520003311
Dumitrascu, Branje, Hladkowicz, Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis, J Am Geriatr Soc, doi:10.1111/jgs.17299
Folsom, Jacobs, Caspersen, Test-retest reliability of the Minnesota leisure time physical activity questionnaire, J Chronic Dis, doi:10.1016/0021-9681(86)90195-5
Fried, Tangen, Walston, Frailty in older adults: evidence for a phenotype, J Gerontol A Biol Med Sci
Geerinck, Locquet, Bruyère, Evaluating quality of life in frailty: applicability and clinimetric properties of the SarQoL ® questionnaire, J Cachexia Sarcopenia Muscle, doi:10.1002/jcsm.12687
Guigoz, Vellas, Nutritional assessment in older adults: MNA ® 25 years of a screening tool and a reference standard for care and research; what next ?, J Nutr Health Aging, doi:10.1007/s12603-021-1601-y
Hanlon, Nicholl, Jani, Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants, Lancet Public Health, doi:10.1016/S2468-2667(18)30091-4
Harvey, Chastin, Skelton, Prevalence of sedentary behavior in older adults: a systematic review, Int J Environ Res Public Health, doi:10.3390/ijerph10126645
Jenkinson, The SF-36 physical and mental health summary measures: an example of how to interpret scores, J Heal Serv Res Policy, doi:10.1177/135581969800300206
Kaiser, Bauer, Ramsch, Validation of the mini nutritional assessment short-form (MNA ® -SF): a practical tool for identification of nutritional status, J Nutr Health Aging, doi:10.1007/s12603-009-0214-7
Kim, Yoon, Kim, Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with coronavirus disease, J Gerontol Ser A, doi:10.1093/gerona/glab085
Landi, Gremese, Bernabei, Post-COVID-19 global health strategies: the need for an interdisciplinary approach, Aging Clin Exp Res, doi:10.1007/s40520-020-01616-x
Leij-Halfwerk, Verwijs, Van Houdt, Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis, Maturitas, doi:10.1016/j.maturitas.2019.05.006
Li, Fan, Lai, Coronavirus infections and immune responses, J Med Virol, doi:10.1002/jmv.25685
Li, Zhang, Gong, Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China, Eur J Clin Nutr, doi:10.1038/s41430-020-0642-3
Lidoriki, Frountzas, Schizas, Could nutritional and functional status serve as prognostic factors for COVID-19 in the elderly?, Med Hypotheses, doi:10.1016/j.mehy.2020.109946
Lingeswaran, Goyal, Ghosh, Inflammation, immunity and immunogenetics in COVID-19: a narrative review, Indian J Clin Biochem, doi:10.1007/s12291-020-00897-3
Ll, Ml, Mm, Cb, Ob; Resources et al., WHO Director-General's opening remarks at the media briefing on COVID-19-11
Mertens, Peñalvo, The burden of malnutrition and fatal COVID-19: a global burden of disease analysis, Front Nutr, doi:10.3389/fnut.2020.619850
Morley, Kalantar-Zadeh, Anker, COVID-19: a major cause of cachexia and sarcopenia?, J Cachexia Sarcopenia Muscle, doi:10.1002/jcsm.12589
Nascimento, Ingles, Sarcopenia, frailty and their prevention by exercise, Free Radic Biol Med, doi:10.1016/j.freeradbiomed.2018.08.035
Negm, Kennedy, Thabane, Management of frailty: a systematic review and network meta-analysis of randomized controlled trials, J Am Med Dir Assoc, doi:10.1016/j.jamda.2019.08.009
Nilsson, Bergens, Kadi, Physical activity alters inflammation in older adults by different intensity levels, Med Sci Sports Exerc, doi:10.1249/MSS.0000000000001582
O'caoimh, Sezgin, Donovan, Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies, Age Ageing, doi:10.1093/ageing/afaa219
Okazaki, Ebihara, Mori, Association between sarcopenia and pneumonia in older people, Geriatr Gerontol Int, doi:10.1111/ggi.13839
Orme, Reis, Herz, Factorial and discriminant validity of the center for epidemiological studies depression (CES-D) scale, J Clin Psychol
Richardson, Leon, Jacobs, Comprehensive evaluation of the Minnesota leisure time physical activity questionnaire, J Clin Epidemiol, doi:10.1016/0895-4356(94)90008-6
Roberts, Denison, Martin, A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach, Age Ageing, doi:10.1093/ageing/afr051
Rubenstein, Harker, Salvà, Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF), J Gerontol, doi:10.1093/gerona/56.6.M366
Salimi, Hamlyn, Couteur, COVID-19 and crosstalk with the hallmarks of aging, J Gerontol, doi:10.1093/gerona/glaa149
Schoevaerdts, Sibille, Gavazzi, Infections in the older population: what do we know?, Aging Clin Exp Res, doi:10.1007/s40520-019-01375-4
Shafiee, Keshtkar, Soltani, Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies, J Diabetes Metab Disord, doi:10.1186/s40200-017-0302-x
Sierra, Bossuyt, Braeye, All-cause mortality supports the COVID-19 mortality in Belgium and comparison with major fatal events of the last century, Arch Public Heal, doi:10.1186/s13690-020-00496-x
Sofra, Badami, Adverse effects of sedentary lifestyles: inflammation, and high-glucose induced oxidative stress-a double blind randomized clinical trial on diabetic and prediabetic patients, Health, doi:10.4236/health.2020.128076
Streicher, Van Zwienen-Pot, Bardon, Determinants of incident malnutrition in community-dwelling older adults: a Manuel multicohort meta-analysis, J Am Geriatr Soc, doi:10.1111/jgs.15553
Suardi, Cazzaniga, Graci, Link between viral infections, immune system, inflammation and diet, Int J Environ Res Public Health, doi:10.3390/ijerph18052455
Taylor, Jacobs, Schucker, A questionnaire for the assessment of leisure time physical activities, J Chronic Dis, doi:10.1016/0021-9681(78)90058-9
Tombaugh, Mcintyre, The Mini-mental state examination: a comprehensive review, J Am Geriatr Soc, doi:10.1111/j.1532-5415.1992.tb01992.x
Vellas, Villars, Abellan, Overview of the MNA ® -its history and challenges, J Nutr Heal Aging
Wang, Li, Wang, Sarcopenia: an underlying treatment target during the COVID-19 pandemic, Nutrition, doi:10.1016/j.nut.2020.111104
Wierdsma, Kruizenga, Konings, Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission, Clin Nutr ESPEN, doi:10.1016/j.clnesp.2021.03.021
Willows, Alam, Sandhu, A Canadian perspective on severe acute respiratory syndrome coronavirus 2 infection and treatment: how prevalent underlying inflammatory disease contributes to pathogenesis, Biochem Cell Biol, doi:10.1139/bcb-2020-0341
Woolford, Angelo, Curtis, COVID-19 and associations with frailty and multimorbidity: a prospective analysis of UK Biobank participants, Aging Clin Exp Res, doi:10.1007/s40520-020-01653-6
Wu, Chen, Cai, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med, doi:10.1001/jamainternmed.2020.0994
Wu, Lewis, Pae, Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance, Front Immunol, doi:10.3389/fimmu.2018.03160
Wyper, Assunção, Cuschieri, Population vulnerability to COVID-19 in Europe: a burden of disease analysis, Arch Public Heal, doi:10.1186/s13690-020-00433-y
Yu, Ye, Chen, Erratum to: malnutrition prolongs the hospitalization of patients with COVID-19 infection: a clinical epidemiological analysis (The journal of nutrition, health and aging, J Nutr Heal Aging
Zadak, Hyspler, Ticha, Polypharmacy and malnutrition, Curr Opin Clin Nutr Metab Care, doi:10.1097/MCO.0b013e32835b612e
Zhang, Zhou, Qiu, Clinical characteristics of 82 death cases with COVID-19, doi:10.1101/2020.02.26.20028191
Zucchelli, Bologna, Marengoni, Why data on frailty and SARS-CoV-2 infection are basic to progress, Aging Clin Exp Res, doi:10.1007/s40520-021-01846-7
DOI record:
{
"DOI": "10.1007/s40520-021-01991-z",
"ISSN": [
"1720-8319"
],
"URL": "http://dx.doi.org/10.1007/s40520-021-01991-z",
"alternative-id": [
"1991"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "16 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "25 September 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "23 October 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no conflict of interest."
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The guidelines of the Declaration of Helsinki were followed, and the present study was approved by the ethics committee of the University of Liege Teaching Hospital (reference 2012/277), with two amendments in 2015 and 2018."
},
{
"group": {
"label": "Human and animal rights",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments."
},
{
"group": {
"label": "Consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "Informed consent was obtained from all participants involved in the study."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 6,
"value": "Informed consent was obtained from all participants involved in the study."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5139-3591",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lengelé",
"given": "Laetitia",
"sequence": "first"
},
{
"affiliation": [],
"family": "Locquet",
"given": "Médéa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moutschen",
"given": "Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beaudart",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaux",
"given": "Jean-François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gillain",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reginster",
"given": "Jean-Yves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruyère",
"given": "Olivier",
"sequence": "additional"
}
],
"container-title": "Aging Clinical and Experimental Research",
"container-title-short": "Aging Clin Exp Res",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
23
]
],
"date-time": "2021-10-23T17:02:31Z",
"timestamp": 1635008551000
},
"deposited": {
"date-parts": [
[
2022,
1,
27
]
],
"date-time": "2022-01-27T10:27:43Z",
"timestamp": 1643279263000
},
"funder": [
{
"DOI": "10.13039/501100002661",
"doi-asserted-by": "publisher",
"name": "fonds de la recherche scientifique - fnrs"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
15
]
],
"date-time": "2022-04-15T08:27:17Z",
"timestamp": 1650011237829
},
"is-referenced-by-count": 2,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
10,
23
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
23
]
],
"date-time": "2021-10-23T00:00:00Z",
"timestamp": 1634947200000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
23
]
],
"date-time": "2021-10-23T00:00:00Z",
"timestamp": 1634947200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40520-021-01991-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40520-021-01991-z/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40520-021-01991-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "223-234",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2021,
10,
23
]
]
},
"published-online": {
"date-parts": [
[
2021,
10,
23
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "1991_CR1",
"unstructured": "World Health Organization (WHO) (2020) WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020"
},
{
"DOI": "10.1016/j.archger.2020.104124",
"author": "LK Chen",
"doi-asserted-by": "publisher",
"journal-title": "Arch Gerontol Geriatr",
"key": "1991_CR2",
"unstructured": "Chen LK (2020) Older adults and COVID-19 pandemic: resilience matters. Arch Gerontol Geriatr 89:104124. https://doi.org/10.1016/j.archger.2020.104124",
"volume": "89",
"year": "2020"
},
{
"DOI": "10.46945/bpj.10.1.03.01",
"doi-asserted-by": "crossref",
"key": "1991_CR3",
"unstructured": "Belgium: WHO Coronavirus Disease (COVID-19) Dashboard With Vaccination Data|WHO Coronavirus (COVID-19) Dashboard With Vaccination Data Available online: https://covid19.who.int/region/euro/country/be. Accessed on 10 Jul 2021"
},
{
"DOI": "10.1139/bcb-2020-0341",
"author": "S Willows",
"doi-asserted-by": "publisher",
"first-page": "173",
"journal-title": "Biochem Cell Biol",
"key": "1991_CR4",
"unstructured": "Willows S, Alam SB, Sandhu JK et al (2021) A Canadian perspective on severe acute respiratory syndrome coronavirus 2 infection and treatment: how prevalent underlying inflammatory disease contributes to pathogenesis. Biochem Cell Biol 99:173–194. https://doi.org/10.1139/bcb-2020-0341",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1002/jmv.25685",
"author": "G Li",
"doi-asserted-by": "publisher",
"first-page": "424",
"journal-title": "J Med Virol",
"key": "1991_CR5",
"unstructured": "Li G, Fan Y, Lai Y et al (2020) Coronavirus infections and immune responses. J Med Virol 92:424–432. https://doi.org/10.1002/jmv.25685",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1101/2020.02.26.20028191",
"author": "B Zhang",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "1991_CR6",
"unstructured": "Zhang B, Zhou X, Qiu Y et al (2020) Clinical characteristics of 82 death cases with COVID-19. medRxiv. https://doi.org/10.1101/2020.02.26.20028191",
"year": "2020"
},
{
"DOI": "10.1007/s12291-020-00897-3",
"author": "M Lingeswaran",
"doi-asserted-by": "publisher",
"first-page": "260",
"journal-title": "Indian J Clin Biochem",
"key": "1991_CR7",
"unstructured": "Lingeswaran M, Goyal T, Ghosh R et al (2020) Inflammation, immunity and immunogenetics in COVID-19: a narrative review. Indian J Clin Biochem 35:260–273. https://doi.org/10.1007/s12291-020-00897-3",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1007/s40520-019-01375-4",
"author": "D Schoevaerdts",
"doi-asserted-by": "publisher",
"first-page": "689",
"journal-title": "Aging Clin Exp Res",
"key": "1991_CR8",
"unstructured": "Schoevaerdts D, Sibille FX, Gavazzi G (2021) Infections in the older population: what do we know? Aging Clin Exp Res 33:689–701. https://doi.org/10.1007/s40520-019-01375-4",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1007/s11357-020-00218-9",
"author": "L Bencivenga",
"doi-asserted-by": "publisher",
"first-page": "1089",
"journal-title": "Geroscience",
"key": "1991_CR9",
"unstructured": "Bencivenga L, Rengo G, Varricchi G (2020) Elderly at time of COronaVIrus disease 2019 (COVID-19): possible role of immunosenescence and malnutrition. Geroscience 42:1089–1092. https://doi.org/10.1007/s11357-020-00218-9",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1093/gerona/glaa149",
"author": "S Salimi",
"doi-asserted-by": "publisher",
"first-page": "e34",
"journal-title": "J Gerontol",
"key": "1991_CR10",
"unstructured": "Salimi S, Hamlyn JM, Le Couteur D (2020) COVID-19 and crosstalk with the hallmarks of aging. J Gerontol 75:e34–e41. https://doi.org/10.1093/gerona/glaa149",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.3389/fphys.2020.571416",
"author": "V Bajaj",
"doi-asserted-by": "publisher",
"first-page": "1793",
"journal-title": "Front Physiol",
"key": "1991_CR11",
"unstructured": "Bajaj V, Gadi N, Spihlman AP et al (2021) Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol 11:1793. https://doi.org/10.3389/fphys.2020.571416",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"author": "C Wu",
"doi-asserted-by": "publisher",
"first-page": "934",
"journal-title": "JAMA Intern Med",
"key": "1991_CR12",
"unstructured": "Wu C, Chen X, Cai Y et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:934–943. https://doi.org/10.1001/jamainternmed.2020.0994",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1016/j.clnu.2016.09.004",
"author": "T Cederholm",
"doi-asserted-by": "publisher",
"first-page": "49",
"journal-title": "Clin Nutr",
"key": "1991_CR13",
"unstructured": "Cederholm T, Barazzoni R, Austin P et al (2017) ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 36:49–64. https://doi.org/10.1016/j.clnu.2016.09.004",
"volume": "36",
"year": "2017"
},
{
"DOI": "10.1017/S0007114520003311",
"author": "AH De Araújo Morais",
"doi-asserted-by": "publisher",
"first-page": "851",
"journal-title": "Br J Nutr",
"key": "1991_CR14",
"unstructured": "De Araújo Morais AH, Aquino JDS, Da Silva-Maia JK et al (2021) Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br J Nutr 125:851–862. https://doi.org/10.1017/S0007114520003311",
"volume": "125",
"year": "2021"
},
{
"DOI": "10.3390/ijerph18052455",
"author": "C Suardi",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Int J Environ Res Public Health",
"key": "1991_CR15",
"unstructured": "Suardi C, Cazzaniga E, Graci S et al (2021) Link between viral infections, immune system, inflammation and diet. Int J Environ Res Public Health 18:1–13. https://doi.org/10.3390/ijerph18052455",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.clnu.2020.03.022",
"author": "R Barazzoni",
"doi-asserted-by": "publisher",
"first-page": "1631",
"journal-title": "Clin Nutr",
"key": "1991_CR16",
"unstructured": "Barazzoni R, Bischoff SC, Breda J et al (2020) ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr 39:1631–1638. https://doi.org/10.1016/j.clnu.2020.03.022",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1007/s40520-020-01616-x",
"author": "F Landi",
"doi-asserted-by": "publisher",
"first-page": "1613",
"journal-title": "Aging Clin Exp Res",
"key": "1991_CR17",
"unstructured": "Landi F, Gremese E, Bernabei R et al (2020) Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res 32:1613–1620. https://doi.org/10.1007/s40520-020-01616-x",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2018.03160",
"author": "D Wu",
"doi-asserted-by": "publisher",
"first-page": "3160",
"journal-title": "Front Immunol",
"key": "1991_CR18",
"unstructured": "Wu D, Lewis ED, Pae M et al (2019) Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol 10:3160. https://doi.org/10.3389/fimmu.2018.03160",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1007/s40520-021-01846-7",
"author": "A Zucchelli",
"doi-asserted-by": "publisher",
"first-page": "1429",
"journal-title": "Aging Clin Exp Res",
"key": "1991_CR19",
"unstructured": "Zucchelli A, Bologna E, Marengoni A (2021) Why data on frailty and SARS-CoV-2 infection are basic to progress. Aging Clin Exp Res 33:1429–1432. https://doi.org/10.1007/s40520-021-01846-7",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2020.109946",
"author": "I Lidoriki",
"doi-asserted-by": "publisher",
"journal-title": "Med Hypotheses",
"key": "1991_CR20",
"unstructured": "Lidoriki I, Frountzas M, Schizas D (2020) Could nutritional and functional status serve as prognostic factors for COVID-19 in the elderly? Med Hypotheses 144:109946. https://doi.org/10.1016/j.mehy.2020.109946",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(12)62167-9",
"author": "A Clegg",
"doi-asserted-by": "publisher",
"first-page": "752",
"journal-title": "Lancet",
"key": "1991_CR21",
"unstructured": "Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. Lancet 381:752–762. https://doi.org/10.1016/S0140-6736(12)62167-9",
"volume": "381",
"year": "2013"
},
{
"DOI": "10.1093/ageing/afy169",
"author": "AJ Cruz-Jentoft",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Age Ageing",
"key": "1991_CR22",
"unstructured": "Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169",
"volume": "48",
"year": "2019"
},
{
"DOI": "10.1111/ggi.13839",
"author": "T Okazaki",
"doi-asserted-by": "publisher",
"first-page": "7",
"journal-title": "Geriatr Gerontol Int",
"key": "1991_CR23",
"unstructured": "Okazaki T, Ebihara S, Mori T et al (2020) Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int 20:7–13. https://doi.org/10.1111/ggi.13839",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.nut.2020.111104",
"author": "P-Y Wang",
"doi-asserted-by": "publisher",
"journal-title": "Nutrition",
"key": "1991_CR24",
"unstructured": "Wang P-Y, Li Y, Wang Q (2021) Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition. https://doi.org/10.1016/j.nut.2020.111104",
"year": "2021"
},
{
"DOI": "10.1016/j.maturitas.2019.05.006",
"author": "S Leij-Halfwerk",
"doi-asserted-by": "publisher",
"first-page": "80",
"journal-title": "Maturitas",
"key": "1991_CR25",
"unstructured": "Leij-Halfwerk S, Verwijs MH, van Houdt S et al (2019) Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis. Maturitas 126:80–89. https://doi.org/10.1016/j.maturitas.2019.05.006",
"volume": "126",
"year": "2019"
},
{
"DOI": "10.1093/ageing/afaa219",
"author": "R O’Caoimh",
"doi-asserted-by": "publisher",
"first-page": "96",
"journal-title": "Age Ageing",
"key": "1991_CR26",
"unstructured": "O’Caoimh R, Sezgin D, O’Donovan MR et al (2021) Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing 50:96–104. https://doi.org/10.1093/ageing/afaa219",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1186/s40200-017-0302-x",
"author": "G Shafiee",
"doi-asserted-by": "publisher",
"journal-title": "J Diabetes Metab Disord",
"key": "1991_CR27",
"unstructured": "Shafiee G, Keshtkar A, Soltani A et al (2017) Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. https://doi.org/10.1186/s40200-017-0302-x",
"year": "2017"
},
{
"DOI": "10.1007/s40520-020-01653-6",
"author": "SJ Woolford",
"doi-asserted-by": "publisher",
"first-page": "1897",
"journal-title": "Aging Clin Exp Res",
"key": "1991_CR28",
"unstructured": "Woolford SJ, D’Angelo S, Curtis EM et al (2020) COVID-19 and associations with frailty and multimorbidity: a prospective analysis of UK Biobank participants. Aging Clin Exp Res 32:1897–1905. https://doi.org/10.1007/s40520-020-01653-6",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1016/j.jns.2020.117136",
"author": "OH Del Brutto",
"doi-asserted-by": "publisher",
"journal-title": "J Neurol Sci",
"key": "1991_CR29",
"unstructured": "Del Brutto OH, Costa AF, Recalde BY et al (2020) Frailty and SARS-CoV-2 infection. A population-based study in a highly endemic village. J Neurol Sci. https://doi.org/10.1016/j.jns.2020.117136",
"year": "2020"
},
{
"DOI": "10.1016/j.exger.2015.05.003",
"author": "C Beaudart",
"doi-asserted-by": "publisher",
"first-page": "103",
"journal-title": "Exp Gerontol",
"key": "1991_CR30",
"unstructured": "Beaudart C, Reginster JY, Petermans J et al (2015) Quality of life and physical components linked to sarcopenia: the SarcoPhAge study. Exp Gerontol 69:103–110. https://doi.org/10.1016/j.exger.2015.05.003",
"volume": "69",
"year": "2015"
},
{
"DOI": "10.4081/monaldi.2012.121",
"author": "G Balestroni",
"doi-asserted-by": "publisher",
"first-page": "155",
"journal-title": "Monaldi Arch Chest Dis",
"key": "1991_CR31",
"unstructured": "Balestroni G, Bertolotti G (2015) EuroQol-5D (EQ-5D): an instrument for measuring quality of life. Monaldi Arch Chest Dis 78:155–159. https://doi.org/10.4081/monaldi.2012.121",
"volume": "78",
"year": "2015"
},
{
"DOI": "10.1002/jcsm.12687",
"author": "A Geerinck",
"doi-asserted-by": "publisher",
"journal-title": "J Cachexia Sarcopenia Muscle",
"key": "1991_CR32",
"unstructured": "Geerinck A, Locquet M, Bruyère O et al (2021) Evaluating quality of life in frailty: applicability and clinimetric properties of the SarQoL® questionnaire. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.12687",
"year": "2021"
},
{
"DOI": "10.1177/135581969800300206",
"author": "C Jenkinson",
"doi-asserted-by": "publisher",
"first-page": "92",
"journal-title": "J Heal Serv Res Policy",
"key": "1991_CR33",
"unstructured": "Jenkinson C (1998) The SF-36 physical and mental health summary measures: an example of how to interpret scores. J Heal Serv Res Policy 3:92–96. https://doi.org/10.1177/135581969800300206",
"volume": "3",
"year": "1998"
},
{
"key": "1991_CR34",
"unstructured": "Nestlé Nutrition Institute Nutrition screening as easy as MNA: a guide to complete the Mini Nutiritonal Assessment (MNA). Available online: https://www.mna-elderly.com/forms/mna_guide_english.pdf. Accessed on 17 Feb 2021"
},
{
"author": "B Vellas",
"first-page": "456",
"journal-title": "J Nutr Heal Aging",
"key": "1991_CR35",
"unstructured": "Vellas B, Villars H, Abellan G et al (2006) Overview of the MNA®—its history and challenges. J Nutr Heal Aging 10:456–463",
"volume": "10",
"year": "2006"
},
{
"DOI": "10.1007/s12603-009-0214-7",
"author": "MJ Kaiser",
"doi-asserted-by": "publisher",
"journal-title": "J Nutr Health Aging",
"key": "1991_CR36",
"unstructured": "Kaiser MJ, Bauer JM, Ramsch C et al (2009) Validation of the mini nutritional assessment short-form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. https://doi.org/10.1007/s12603-009-0214-7",
"year": "2009"
},
{
"DOI": "10.1093/gerona/56.6.M366",
"author": "LZ Rubenstein",
"doi-asserted-by": "publisher",
"first-page": "366",
"journal-title": "J Gerontol",
"key": "1991_CR37",
"unstructured": "Rubenstein LZ, Harker JO, Salvà A et al (2001) Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol 56:366–372. https://doi.org/10.1093/gerona/56.6.M366",
"volume": "56",
"year": "2001"
},
{
"DOI": "10.1177/0036850419854290",
"author": "C Corcoran",
"doi-asserted-by": "publisher",
"first-page": "171",
"journal-title": "Sci Prog",
"key": "1991_CR38",
"unstructured": "Corcoran C, Murphy C, Culligan EP et al (2019) Malnutrition in the elderly. Sci Prog 102:171–180. https://doi.org/10.1177/0036850419854290",
"volume": "102",
"year": "2019"
},
{
"DOI": "10.1007/s12603-021-1601-y",
"author": "Y Guigoz",
"doi-asserted-by": "publisher",
"journal-title": "J Nutr Health Aging",
"key": "1991_CR39",
"unstructured": "Guigoz Y, Vellas B (2021) Nutritional assessment in older adults: MNA® 25 years of a screening tool and a reference standard for care and research; what next ? J Nutr Health Aging. https://doi.org/10.1007/s12603-021-1601-y",
"year": "2021"
},
{
"DOI": "10.1093/ageing/afr051",
"author": "HC Roberts",
"doi-asserted-by": "publisher",
"first-page": "423",
"journal-title": "Age Ageing",
"key": "1991_CR40",
"unstructured": "Roberts HC, Denison HJ, Martin HJ et al (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40:423–429. https://doi.org/10.1093/ageing/afr051",
"volume": "40",
"year": "2011"
},
{
"DOI": "10.1093/gerona/56.3.M146",
"author": "LP Fried",
"doi-asserted-by": "publisher",
"first-page": "146",
"journal-title": "J Gerontol A Biol Med Sci",
"key": "1991_CR41",
"unstructured": "Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Med Sci 56:146–156",
"volume": "56",
"year": "2001"
},
{
"DOI": "10.1002/1097-4679(198601)42:1<28::AID-JCLP2270420104>3.0.CO;2-T",
"author": "JG Orme",
"doi-asserted-by": "publisher",
"first-page": "28",
"journal-title": "J Clin Psychol",
"key": "1991_CR42",
"unstructured": "Orme JG, Reis J, Herz EJ (1984) Factorial and discriminant validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol 42:28–33",
"volume": "42",
"year": "1984"
},
{
"DOI": "10.1016/0021-9681(78)90058-9",
"author": "HL Taylor",
"doi-asserted-by": "publisher",
"first-page": "741",
"journal-title": "J Chronic Dis",
"key": "1991_CR43",
"unstructured": "Taylor HL, Jacobs DR, Schucker B et al (1978) A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31:741–755. https://doi.org/10.1016/0021-9681(78)90058-9",
"volume": "31",
"year": "1978"
},
{
"DOI": "10.1097/MCO.0b013e32835b612e",
"author": "Z Zadak",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Curr Opin Clin Nutr Metab Care",
"key": "1991_CR44",
"unstructured": "Zadak Z, Hyspler R, Ticha A et al (2013) Polypharmacy and malnutrition. Curr Opin Clin Nutr Metab Care 16:50–55. https://doi.org/10.1097/MCO.0b013e32835b612e",
"volume": "16",
"year": "2013"
},
{
"DOI": "10.1111/jgs.15553",
"author": "M Streicher",
"doi-asserted-by": "publisher",
"first-page": "2335",
"journal-title": "J Am Geriatr Soc",
"key": "1991_CR45",
"unstructured": "Streicher M, van Zwienen-Pot J, Bardon L et al (2018) Determinants of incident malnutrition in community-dwelling older adults: a Manuel multicohort meta-analysis. J Am Geriatr Soc 66:2335–2343. https://doi.org/10.1111/jgs.15553",
"volume": "66",
"year": "2018"
},
{
"DOI": "10.1016/j.clnu.2013.05.007",
"author": "V Bokhorst-de",
"doi-asserted-by": "publisher",
"journal-title": "Clin Nutr",
"key": "1991_CR46",
"unstructured": "Bokhorst-de V, van der Schueren MAE, Lonterman-Monasch S et al (2013) Prevalence and determinants for malnutrition in geriatric outpatients. Clin Nutr. https://doi.org/10.1016/j.clnu.2013.05.007",
"year": "2013"
},
{
"DOI": "10.3389/fnagi.2015.00056",
"author": "T Coelho",
"doi-asserted-by": "publisher",
"journal-title": "Front Aging Neurosci",
"key": "1991_CR47",
"unstructured": "Coelho T, Paúl C, Gobbens RJJ et al (2015) Determinants of frailty: The added value of assessing medication. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2015.00056",
"year": "2015"
},
{
"DOI": "10.1111/j.1532-5415.1992.tb01992.x",
"author": "TN Tombaugh",
"doi-asserted-by": "publisher",
"first-page": "922",
"journal-title": "J Am Geriatr Soc",
"key": "1991_CR48",
"unstructured": "Tombaugh TN, McIntyre NJ (1992) The Mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40:922–935. https://doi.org/10.1111/j.1532-5415.1992.tb01992.x",
"volume": "40",
"year": "1992"
},
{
"DOI": "10.1016/j.clnesp.2021.03.002",
"author": "SM Abate",
"doi-asserted-by": "publisher",
"first-page": "174",
"journal-title": "Clin Nutr ESPEN",
"key": "1991_CR49",
"unstructured": "Abate SM, Chekole YA, Estifanos MB et al (2021) Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: a systematic review and meta-analysis. Clin Nutr ESPEN 43:174–183. https://doi.org/10.1016/j.clnesp.2021.03.002",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1016/j.clnesp.2021.03.021",
"author": "NJ Wierdsma",
"doi-asserted-by": "publisher",
"first-page": "369",
"journal-title": "Clin Nutr ESPEN",
"key": "1991_CR50",
"unstructured": "Wierdsma NJ, Kruizenga HM, Konings LA et al (2021) Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN 43:369–376. https://doi.org/10.1016/j.clnesp.2021.03.021",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1002/jcsm.12589",
"author": "JE Morley",
"doi-asserted-by": "publisher",
"first-page": "863",
"journal-title": "J Cachexia Sarcopenia Muscle",
"key": "1991_CR51",
"unstructured": "Morley JE, Kalantar-Zadeh K, Anker SD (2020) COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle 11:863–865. https://doi.org/10.1002/jcsm.12589",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41430-020-0642-3",
"author": "T Li",
"doi-asserted-by": "publisher",
"first-page": "871",
"journal-title": "Eur J Clin Nutr",
"key": "1991_CR52",
"unstructured": "Li T, Zhang Y, Gong C et al (2020) Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr 74:871–875. https://doi.org/10.1038/s41430-020-0642-3",
"volume": "74",
"year": "2020"
},
{
"DOI": "10.1007/s12603-020-1541-y",
"author": "Y Yu",
"doi-asserted-by": "publisher",
"first-page": "369",
"journal-title": "J Nutr Heal Aging",
"key": "1991_CR53",
"unstructured": "Yu Y, Ye J, Chen M et al (2021) Erratum to: malnutrition prolongs the hospitalization of patients with COVID-19 infection: a clinical epidemiological analysis (The journal of nutrition, health and aging, (2020), 10.1007/s12603-020-1541-y). J Nutr Heal Aging 25:369–373",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.3389/fnut.2020.619850",
"author": "E Mertens",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Front Nutr",
"key": "1991_CR54",
"unstructured": "Mertens E, Peñalvo JL (2021) The burden of malnutrition and fatal COVID-19: a global burden of disease analysis. Front Nutr 7:1–12. https://doi.org/10.3389/fnut.2020.619850",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1093/gerona/glab085",
"author": "J-W Kim",
"doi-asserted-by": "publisher",
"journal-title": "J Gerontol Ser A",
"key": "1991_CR55",
"unstructured": "Kim J-W, Yoon JS, Kim EJ et al (2021) Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with coronavirus disease 2019. J Gerontol Ser A. https://doi.org/10.1093/gerona/glab085",
"year": "2021"
},
{
"DOI": "10.1016/S2468-2667(18)30091-4",
"author": "P Hanlon",
"doi-asserted-by": "publisher",
"first-page": "e323",
"journal-title": "Lancet Public Health",
"key": "1991_CR56",
"unstructured": "Hanlon P, Nicholl BI, Jani BD et al (2018) Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 3:e323–e332. https://doi.org/10.1016/S2468-2667(18)30091-4",
"volume": "3",
"year": "2018"
},
{
"DOI": "10.1186/s13690-020-00433-y",
"author": "GMA Wyper",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Arch Public Heal",
"key": "1991_CR57",
"unstructured": "Wyper GMA, Assunção R, Cuschieri S et al (2020) Population vulnerability to COVID-19 in Europe: a burden of disease analysis. Arch Public Heal 78:1–8. https://doi.org/10.1186/s13690-020-00433-y",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1186/s13690-020-00496-x",
"author": "N Bustos Sierra",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Arch Public Heal",
"key": "1991_CR58",
"unstructured": "Bustos Sierra N, Bossuyt N, Braeye T et al (2020) All-cause mortality supports the COVID-19 mortality in Belgium and comparison with major fatal events of the last century. Arch Public Heal 78:1–8. https://doi.org/10.1186/s13690-020-00496-x",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1111/jgs.17299",
"author": "F Dumitrascu",
"doi-asserted-by": "publisher",
"journal-title": "J Am Geriatr Soc",
"key": "1991_CR59",
"unstructured": "Dumitrascu F, Branje KE, Hladkowicz ES et al (2021) Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J Am Geriatr Soc. https://doi.org/10.1111/jgs.17299",
"year": "2021"
},
{
"DOI": "10.1159/000382063",
"author": "T Cederholm",
"doi-asserted-by": "publisher",
"first-page": "65",
"journal-title": "Nestle Nutr Inst Workshop Ser",
"key": "1991_CR60",
"unstructured": "Cederholm T (2015) Overlaps between frailty and sarcopenia definitions. Nestle Nutr Inst Workshop Ser 83:65–69. https://doi.org/10.1159/000382063",
"volume": "83",
"year": "2015"
},
{
"DOI": "10.1016/j.freeradbiomed.2018.08.035",
"author": "CM Nascimento",
"doi-asserted-by": "publisher",
"first-page": "42",
"journal-title": "Free Radic Biol Med",
"key": "1991_CR61",
"unstructured": "Nascimento CM, Ingles M, Salvador-Pascual A et al (2019) Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med 132:42–49. https://doi.org/10.1016/j.freeradbiomed.2018.08.035",
"volume": "132",
"year": "2019"
},
{
"DOI": "10.4236/health.2020.128076",
"author": "X Sofra",
"doi-asserted-by": "publisher",
"first-page": "1029",
"journal-title": "Health (Irvine Calif)",
"key": "1991_CR62",
"unstructured": "Sofra X, Badami S (2020) Adverse effects of sedentary lifestyles: inflammation, and high-glucose induced oxidative stress—a double blind randomized clinical trial on diabetic and prediabetic patients. Health (Irvine Calif) 12:1029–1048. https://doi.org/10.4236/health.2020.128076",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1249/MSS.0000000000001582",
"author": "A Nilsson",
"doi-asserted-by": "publisher",
"first-page": "1502",
"journal-title": "Med Sci Sports Exerc",
"key": "1991_CR63",
"unstructured": "Nilsson A, Bergens O, Kadi F (2018) Physical activity alters inflammation in older adults by different intensity levels. Med Sci Sports Exerc 50:1502–1507. https://doi.org/10.1249/MSS.0000000000001582",
"volume": "50",
"year": "2018"
},
{
"DOI": "10.1007/s10238-020-00650-3",
"author": "MP da Silveira",
"doi-asserted-by": "publisher",
"first-page": "15",
"journal-title": "Clin Exp Med",
"key": "1991_CR64",
"unstructured": "da Silveira MP, da Silva Fagundes KK, Bizuti MR et al (2021) Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin Exp Med 21:15–28. https://doi.org/10.1007/s10238-020-00650-3",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.3390/ijerph10126645",
"author": "JA Harvey",
"doi-asserted-by": "publisher",
"first-page": "6645",
"journal-title": "Int J Environ Res Public Health",
"key": "1991_CR65",
"unstructured": "Harvey JA, Chastin SFM, Skelton DA (2013) Prevalence of sedentary behavior in older adults: a systematic review. Int J Environ Res Public Health 10:6645–6661. https://doi.org/10.3390/ijerph10126645",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1016/j.jamda.2019.08.009",
"author": "AM Negm",
"doi-asserted-by": "publisher",
"first-page": "1190",
"journal-title": "J Am Med Dir Assoc",
"key": "1991_CR66",
"unstructured": "Negm AM, Kennedy CC, Thabane L et al (2019) Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J Am Med Dir Assoc 20:1190–1198. https://doi.org/10.1016/j.jamda.2019.08.009",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1016/S1473-3099(21)00460-6",
"author": "M Antonelli",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "1991_CR67",
"unstructured": "Antonelli M, Penfold RS, Merino J et al (2021) Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(21)00460-6",
"year": "2021"
},
{
"DOI": "10.1016/0021-9681(86)90195-5",
"author": "AR Folsom",
"doi-asserted-by": "publisher",
"first-page": "505",
"journal-title": "J Chronic Dis",
"key": "1991_CR68",
"unstructured": "Folsom AR, Jacobs DR, Caspersen CJ et al (1986) Test-retest reliability of the Minnesota leisure time physical activity questionnaire. J Chronic Dis 39:505–511. https://doi.org/10.1016/0021-9681(86)90195-5",
"volume": "39",
"year": "1986"
},
{
"DOI": "10.1016/0895-4356(94)90008-6",
"author": "MT Richardson",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "J Clin Epidemiol",
"key": "1991_CR69",
"unstructured": "Richardson MT, Leon AS, Jacobs DR et al (1994) Comprehensive evaluation of the Minnesota leisure time physical activity questionnaire. J Clin Epidemiol 47:271–281. https://doi.org/10.1016/0895-4356(94)90008-6",
"volume": "47",
"year": "1994"
}
],
"reference-count": 69,
"references-count": 69,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40520-021-01991-z"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Geriatrics and Gerontology",
"Aging"
],
"subtitle": [],
"title": "Frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19 in older community-dwelling adults",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "34"
}
