Effect of Short Term High Dose Oral Vitamin D Therapy on the Inflammatory Markers in Patients with COVID 19 Disease
et al., Archives of Clinical and Biomedical Research, doi:10.26502/acbr.50170273, Jul 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 44 treatment and 43 control patients with vitamin D levels <30ng/ml, showing significant reduction in inflammatory markers with treatment of 60,000IU vitamin D per day for 8 days (10 days for BMI >25). Death and ICU admission was lower in the treatment group but not statistically significant. Randomization was simple alternation, with the allocation officer unaware of which group patients were being assigned to as detailed in the study.
An earlier version of this study was censored based on incorrect claims from an anti-treatment researcher. For discussion see1.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 26th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 97th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of death, 60.9% lower, RR 0.39, p = 0.27, treatment 2 of 44 (4.5%), control 5 of 43 (11.6%), NNT 14.
|
|
risk of ICU admission, 21.8% lower, RR 0.78, p = 0.74, treatment 4 of 44 (9.1%), control 5 of 43 (11.6%), NNT 39.
|
|
hospitalization time, 7.1% lower, relative time 0.93, p = 0.90, treatment 44, control 43.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lakkireddy et al., 27 Jul 2022, Randomized Controlled Trial, India, peer-reviewed, mean age 45.5, 9 authors, dosage 60,000IU days 1-8, 8 or 10 days depending on BMI.
Effect of Short Term High Dose Oral Vitamin D Therapy on the Inflammatory Markers in Patients with COVID 19 Disease
Archives of Clinical and Biomedical Research, doi:10.26502/acbr.50170273
Introduction: COVID 19 is known to cause immune dysregulation and vitamin D is a known immunomodulator. This study aims to objectively investigate the effect of short term high dose vitamin D therapy in reducing the inflammatory markers of COVID-19.
Materials and Methods: Consented COVID-19 patients with hypovitaminosis D were evaluated for inflammatory markers (N/L ratio, CRP, LDH, IL6, Ferritin) along with vitamin D on 0 th day and 9 th / 11 th day as per their respective BMI category. Subjects were allotted to VD and NVD groups. VD group received Pulse D therapy (a short term high dose supplementation of 60,000 IUs of vitamin D for 8 or 10 days depending upon their BMI) in addition to the standard treatment. NVD group received standard treatment alone. Differences in the variables between the two groups were analysed for statistical significance.
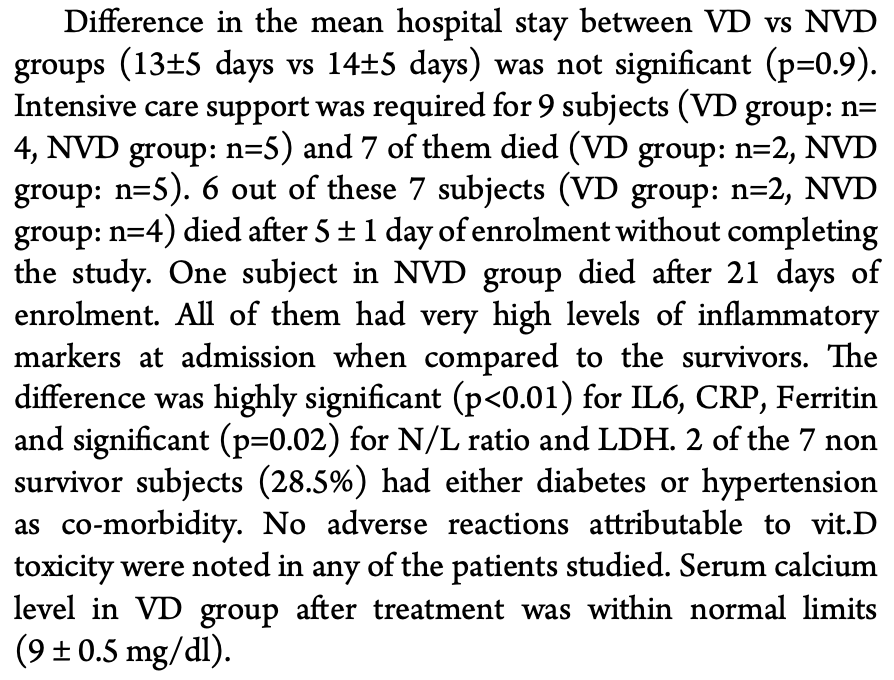
Results: Eighty seven out of one hundred and thirty subjects have completed the study (VD:44, NVD:43). Vitamin D level has increased from 15 ng/ml to 81 ng/ml in VD group and highly significant (p<0.01) reduction of all the measured inflammatory markers was noted. Reduction of markers in NVD group was insignificant (p>0.05). The difference in the reduction of markers between the groups (NVD vs VD) was highly significant (p<0.01). Conclusions: Therapeutic improvement in vitamin D to 80-100 ng/ml has significantly reduced the inflammatory markers associated with COVID-19 without any side effects. Hence, adjunctive short term high dose vitamin D therapy can be added safely to the existing treatment protocols of COVID-19 for improved outcomes.
Declaration of Interest/ Competing Interest None.
Conflict of Interest
Nil
The Author Contributions Statement
References
Adams, Hewison, Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity, Nat Clin Pract Endocrinol Metab
Annweiler, Corvaisier, Gautier, Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients
Aygun, Vitamin D can prevent COVID-19 infection-induced multiple organ damage, Naunyn Schmiedebergs Arch Pharmacol
Baeke, Takiishi, Korf, Vitamin D: modulator of the immune system, Curr Opin Pharmacol
Carpagnano, Lecce, Quaranta, Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19, J Endocrinol Invest
Castillo, Costa, Barrios, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol
Chambers, Hawrylowicz, The impact of vitamin D on regulatory T cells, Curr Allergy Asthma Rep
Chen, Liu, Liu, Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia, Zhonghua Jie He He Hu Xi Za Zhi
Chen, Wu, Guo, Clinical and immunological features of severe and moderate coronavirus disease 2019, J Clin Invest
De Carvalho, Churilov, Safety of megadose of vitamin D in patients with nephrolithiasis, Nutrition
Dinicolantonio, Keefe, Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in covid-19 patients, Mo Med
Ebadi, Montano-Loza, Perspective: improving vitamin D status in the management of COVID-19, Eur J Clin Nutr
Giannini, Passeri, Tripepi, Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study, Nutrients
Gombart, Pierre, Maggini, A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection, Nutrients
Grant, Lahore, Mcdonnell, Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrients
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jain, Chaurasia, Sengar, Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers, Sci Rep
Kaufman, Niles, Kroll, SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels, PLoS One
Lakkireddy, Karra, Patnala, Efficiency of vitamin D supplementation in patients with mechanical low back ache, J Clin Orthop Trauma
Lakkireddy, Srikanth, Gadiga, Malathi, Latha Karra et al., Effect of Short Term High Dose Oral Vitamin D Therapy on the Inflammatory Markers in Patients with COVID 19 Disease, Archives of Clinical and Biomedical Research
Lemire, Adams, Kermani-Arab, 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro, J Immunol
Ling, Broad, Murphy, High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study, Nutrients
Liu, Hong, Yang, A Single Large Dose of Vitamin D Could be Used as a Means of Coronavirus Disease 2019 Prevention and Treatment, Drug Des Devel Ther
Maghbooli, Sahraian, Ebrahimi, Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection, PLoS One
Mariani, Tajer, Antonietti, High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial (CARED-TRIAL), Trials
Mccullough, Lehrer, Amend, Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience, J Steroid Biochem Mol Biol
Mcnally, Iliriani, Pojsupap, Rapid normalization of vitamin D levels: a meta-analysis, Pediatrics
Meltzer, Best, Zhang, Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw Open
Merzon, Tworowski, Gorohovski, Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, FEBS J
Murai, Fernandes, Sales, Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Rastogi, Bhansali, Khare, Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study), Postgrad Med J
Sanders, Monogue, Jodlowski, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review, JAMA
Smet, Smet, Herroelen, Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality, Am J Clin Pathol
Velavan, Meyer, Mild versus severe COVID-19: laboratory markers, Int J Infect Dis
Välimäki, Löyttyniemi, Pekkarinen, How well are the optimal serum 25OHD concentrations reached in high-dose intermittent vitamin D therapy? a placebo-controlled study on comparison between 100 000 IU and 200 000 IU of oral D3 every 3 months in elderly women, Clin Endocrinol (Oxf)
Wu, Camargo, Jr, Reid, What factors modify the effect of monthly bolus dose vitamin D supplementation on 25-hydroxyvitamin D concentrations?, J Steroid Biochem Mol Biol
Ye, Wang, Mao, The pathogenesis and treatment of the `Cytokine Storm' in COVID-19, J Infect
DOI record:
{
"DOI": "10.26502/acbr.50170273",
"ISSN": [
"2572-5017"
],
"URL": "http://dx.doi.org/10.26502/acbr.50170273",
"author": [
{
"affiliation": [],
"family": "Lakkireddy",
"given": "Maheshwar",
"sequence": "first"
},
{
"affiliation": [],
"family": "Goud Gadiga",
"given": "Srikanth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malathi",
"given": "RD",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Latha Karra",
"given": "Madhu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murthy Raju",
"given": "ISSV Prasad",
"sequence": "additional"
},
{
"affiliation": [],
"family": ".",
"given": "Ragini",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chinapaka",
"given": "Sangeetha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baba KSS",
"given": "Sai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kandakatla",
"given": "Manohar",
"sequence": "additional"
}
],
"container-title": "Archives of Clinical and Biomedical Research",
"container-title-short": "Arch Clin Biomed Res",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T03:29:57Z",
"timestamp": 1660102197000
},
"deposited": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T03:29:58Z",
"timestamp": 1660102198000
},
"indexed": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T04:14:20Z",
"timestamp": 1660104860597
},
"is-referenced-by-count": 0,
"issue": "04",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "04",
"published-online": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"member": "11040",
"original-title": [],
"prefix": "10.26502",
"published": {
"date-parts": [
[
2022
]
]
},
"published-online": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Fortune Journals",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.fortunejournals.com/articles/effect-of-short-term-high-dose-oral-vitamin-d-therapy-on-the-inflammatory-markers-in-patients-with-covid-19-disease.html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Linguistics and Language",
"Anthropology",
"History",
"Language and Linguistics",
"Cultural Studies"
],
"subtitle": [],
"title": "Effect of Short Term High Dose Oral Vitamin D Therapy on the Inflammatory Markers in Patients with COVID 19 Disease",
"type": "journal-article",
"volume": "06"
}
