Efficacy of montelukast in the management of COVID-19: double blind randomized placebo controlled trial
et al., International Journal of Basic & Clinical Pharmacology, doi:10.18203/2319-2003.ijbcp20214502, Nov 2021
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0041 from 9 studies.
Lower risk for hospitalization and cases.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
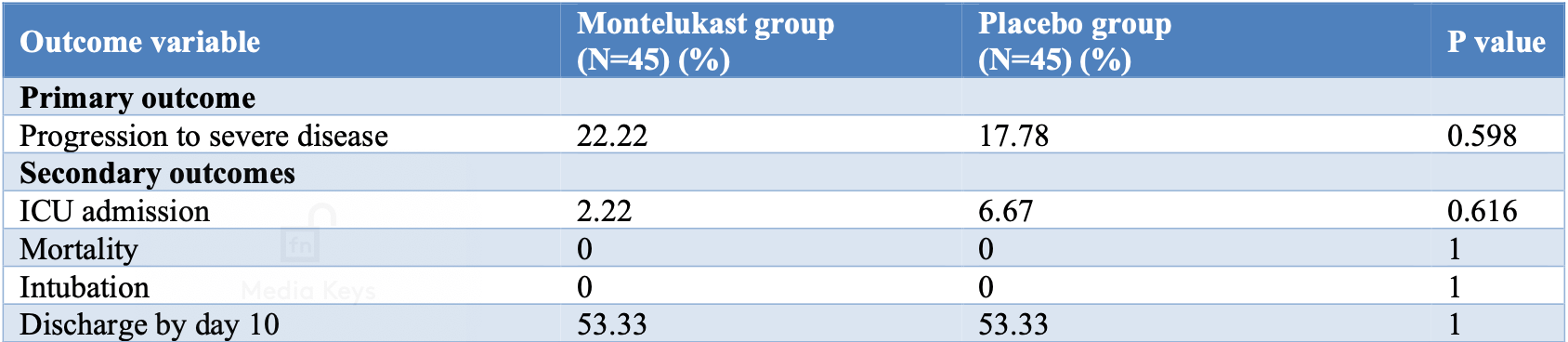
RCT 90 mild to moderate COVID-19 patients showing no significant differences with montelukast treatment.
|
risk of ICU admission, 66.7% lower, RR 0.33, p = 0.62, treatment 1 of 45 (2.2%), control 3 of 45 (6.7%), NNT 23.
|
|
risk of progression, 25.0% higher, RR 1.25, p = 0.79, treatment 10 of 45 (22.2%), control 8 of 45 (17.8%), primary outcome.
|
|
risk of no hospital discharge, no change, RR 1.00, p = 1.00, treatment 21 of 45 (46.7%), control 21 of 45 (46.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kumar et al., 22 Nov 2021, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, mean age 45.0, 10 authors, study period 1 September, 2020 - 31 December, 2020, average treatment delay 5.8 days.
Contact: vk1994@gmail.com.
Efficacy of montelukast in the management of COVID-19: double blind randomized placebo controlled trial
International Journal of Basic & Clinical Pharmacology, doi:10.18203/2319-2003.ijbcp20214502
Background: Objective of the study was to determine the efficacy of montelukast in reducing the severity of COVID-19 symptoms using a double blinded randomized controlled trial. Methods: Parallel, double-blinded randomized controlled trial (RCT) with placebo as comparison to montelukast. All patients above the age of 14 years both males and females, admitted with a diagnosis of mild or moderate COVID-19 (on the basis of a positive reverse transcriptase polymerase chain reaction (RT-PCR) report) at our facility during the study period from 01 September 2020-31 January 2021) and excluding those having adverse reaction to montelukast or those not willing to participate, and pregnant and lactating females. Patients in the intervention arm were given tablet montelukast 10 mg OD HS from the day of admission for 10 days. The patients in the placebo group were given an identical appearing placebo at bedtime for 10 days from the day of admission. The rest of the treatment was given as per the standard operating procedure (SOP) of the institute with minor adjustments as per the treating team's judgement. Primary outcome was progression of the disease to severe grade and secondary outcomes were discharge on or before day 10 from admission, admission to ICU, need for mechanical ventilation and in-hospital mortality. Results: A total of 94 patients were enrolled for the study. 90 patients, 45 in each arm were included in the final analysis. The baseline characteristics of the two arms including age, sex, comorbidities, severity at admission and treatment given apart from montelukast or placebo, were comparable with respect to these variables. This study did not find any improvement in primary outcome of progression to severe disease and secondary outcomes of intensive care unit (ICU) admission, mortality or need of mechanical ventilation, discharge on or by day 10 with the use of montelukast as compared to placebo in mild to moderate cases of COVID-19. Conclusions: There was no difference in primary or secondary outcomes with the use of Montelukast compared to placebo.
References
Barré, Sabatier, Annweiler, Montelukast Drug May Improve COVID-19 Prognosis: A Review of Evidence, Front Pharmacol
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Bozek, Winterstein, Montelukast's ability to fight COVID-19 infection, J Asthma
Cadegiani, Repurposing existing drugs for COVID-19: an endocrinology perspective, BMC Endocr Disord
Chen, Zhang, Pan, Effect of Montelukast on Bronchopulmonary Dysplasia (BPD) and Related Mechanisms, Med Sci Monit Int Med J Exp Clin Res
Echeverría-Esnal, Martin-Ontiyuelo, Navarrete-Rouco, Cuscó, Ferrández et al., Azithromycin in the treatment of COVID-19: a review, Expert Rev Anti Infect Ther
Fidan, Aydoğdu, As a potential treatment of COVID-19: Montelukast, Med Hypotheses
Group, Horby, Pessoa-Amorim, Peto, Brightling et al., Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled
Keeley, Buchanan, Pivodic, Tavabie, Noble, Symptom burden and clinical profile of COVID-19 deaths: a rapid systematic review and evidence summary, BMJ Support Palliat Care
Khan, Misdary, Yegya-Raman, Kim, Narayanan et al., Montelukast in hospitalized patients diagnosed with COVID-19, J Asthma
Lima-Morales, Méndez-Hernández, Flores, Osorno-Romero, Hernández et al., Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico, Int J Infect Dis
López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial, JAMA
Nile, Nile, Qiu, Li, Jia et al., COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons, Cytokine Growth Factor Rev
Noor, Najmi, Bukhtiar, Effect of Montelukast on bradykinin-induced contraction of isolated tracheal smooth muscle of guinea pig, Indian J Pharmacol
Salama, Han, Yau, Reiss, Kramer et al., Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia, N Engl J Med
Tavares, Farraia, Silva, Ribeiro, Severo et al., Impact of montelukast as add on treatment to the novel coronavirus pneumonia (COVID-19): protocol for an investigator-initiated open labeled randomized controlled pragmatic trial, Porto Biomed J
Wilchesky, Repurposing Montelukast for the Attenuation and Prophylaxis of Severe COVID-19 Symptoms: The COvid-19 Symptom MOntelukast (COSMO) Trial
Wu, Chik, Chan, Li, Tsang et al., Anti-inflammatory effects of high-dose montelukast in an animal model of acute asthma, Clin Exp Allergy
Zhu, Zhang, Li, Yang, Song, A Novel Coronavirus from Patients with Pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.18203/2319-2003.ijbcp20214502",
"ISSN": [
"2279-0780",
"2319-2003"
],
"URL": "http://dx.doi.org/10.18203/2319-2003.ijbcp20214502",
"abstract": "<jats:p>Background: Objective of the study was to determine the efficacy of montelukast in reducing the severity of COVID-19 symptoms using a double blinded randomized controlled trial.Methods: Parallel, double-blinded randomized controlled trial (RCT) with placebo as comparison to montelukast. All patients above the age of 14 years both males and females, admitted with a diagnosis of mild or moderate COVID-19 (on the basis of a positive reverse transcriptase polymerase chain reaction (RT-PCR) report) at our facility during the study period from 01 September 2020-31 January 2021) and excluding those having adverse reaction to montelukast or those not willing to participate, and pregnant and lactating females. Patients in the intervention arm were given tablet montelukast 10 mg OD HS from the day of admission for 10 days. The patients in the placebo group were given an identical appearing placebo at bedtime for 10 days from the day of admission. The rest of the treatment was given as per the standard operating procedure (SOP) of the institute with minor adjustments as per the treating team’s judgement. Primary outcome was progression of the disease to severe grade and secondary outcomes were discharge on or before day 10 from admission, admission to ICU, need for mechanical ventilation and in-hospital mortality.Results: A total of 94 patients were enrolled for the study. 90 patients, 45 in each arm were included in the final analysis. The baseline characteristics of the two arms including age, sex, comorbidities, severity at admission and treatment given apart from montelukast or placebo, were comparable with respect to these variables. This study did not find any improvement in primary outcome of progression to severe disease and secondary outcomes of intensive care unit (ICU) admission, mortality or need of mechanical ventilation, discharge on or by day 10 with the use of montelukast as compared to placebo in mild to moderate cases of COVID-19.Conclusions: There was no difference in primary or secondary outcomes with the use of Montelukast compared to placebo.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6042-7784",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kumar",
"given": "Vijay",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ganapule",
"given": "Avinash A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Supriya",
"given": "Sushmita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhushan",
"given": "Divendu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lohani",
"given": "Pallavi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pandey",
"given": "Sanjay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sahoo",
"given": "B. Hilbert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Anjani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Shruti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Ramesh",
"sequence": "additional"
}
],
"container-title": "International Journal of Basic & Clinical Pharmacology",
"container-title-short": "Int J Basic Clin Pharmacol",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
22
]
],
"date-time": "2021-11-22T14:38:53Z",
"timestamp": 1637591933000
},
"deposited": {
"date-parts": [
[
2021,
11,
22
]
],
"date-time": "2021-11-22T14:38:59Z",
"timestamp": 1637591939000
},
"indexed": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T14:40:14Z",
"timestamp": 1648564814048
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2021,
11,
22
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2021,
11,
22
]
]
}
},
"link": [
{
"URL": "https://www.ijbcp.com/index.php/ijbcp/article/viewFile/4867/3323",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.ijbcp.com/index.php/ijbcp/article/viewFile/4867/3323",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "7570",
"original-title": [],
"page": "1374",
"prefix": "10.18203",
"published": {
"date-parts": [
[
2021,
11,
22
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
22
]
]
},
"publisher": "Medip Academy",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ijbcp.com/index.php/ijbcp/article/view/4867"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of montelukast in the management of COVID-19: double blind randomized placebo controlled trial",
"type": "journal-article",
"volume": "10"
}
