Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial
et al., Journal of Family Medicine and Primary Care, doi:10.4103/jfmpc.jfmpc_2437_21, CTRI/2020/11/029230, Aug 2022
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 60 ICU patients in India, showing no significant difference in outcomes with vitamin C. Mortality was lower in the vitamin C arm despite having more severe cases at baseline (87% vs. 67%). 1 gram intravenous vitamin C 8 hourly for four days.
Although the 23% lower mortality is not statistically significant, it is consistent with the significant 18% lower mortality [9‑27%] from meta-analysis of the 45 mortality results to date.
This is the 14th of 21 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0012.
This is the 52nd of 74 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000068.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
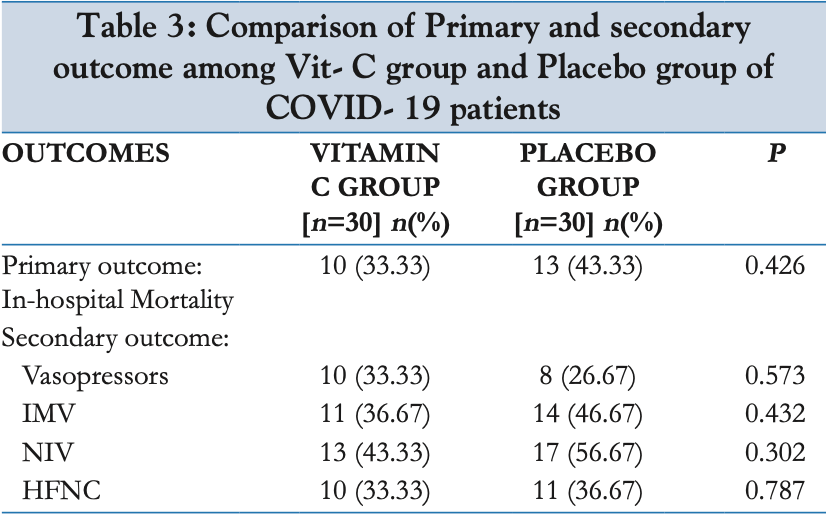
risk of death, 23.1% lower, RR 0.77, p = 0.60, treatment 10 of 30 (33.3%), control 13 of 30 (43.3%), NNT 10.0.
|
|
risk of mechanical ventilation, 21.4% lower, RR 0.79, p = 0.60, treatment 11 of 30 (36.7%), control 14 of 30 (46.7%), NNT 10.0.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kumar et al., 30 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, mean age 57.0, 11 authors, average treatment delay 7.5 days, dosage 1000mg tid days 1-4, trial CTRI/2020/11/029230.
Contact: vk1994@gmail.com.
Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial
Journal of Family Medicine and Primary Care, doi:10.4103/jfmpc.jfmpc_2437_21
Aim: To study the efficacy of intravenous vitamin C in management of moderate and severe COVID-19. Objective: To determine the efficacy of intravenous vitamin C in reducing in-hospital mortality in moderate and severe cases of COVID-19. Design: Parallel, double-blinded randomized controlled trial with placebo. Ethical clearance was obtained from the institutional ethics committee, AIIMS Patna. The trial was registered with the Clinical Trials Registry -India (registration number-CTRI/2020/11/029230.) Setting: A tertiary care centre in Bihar, India Participants: All patients above the age of 18 years both males and females, admitted in ICU with a diagnosis of moderate and severe COVID-19 (on the basis of a positive reverse transcriptase polymerase chain reaction (RT-PCR) report) at our facility during the study period (01/10/2020-31/12/2020) not having any of the exclusion criteria. Intervention: The patients in the intervention arm were given 1 gram (2 ampoules of 2 ml each containing 500 mg of vitamin C mixed in 100 ml normal saline) intravenous vitamin C 8 hourly for four days. The patients in the placebo arm received similar looking ampoules (2 ampoules of 2 ml sterile water for injection mixed in 100 ml normal saline) intravenously 8 hourly for four days. The rest of the treatment was given as per the standard operating procedure (SOP) of the institute with adjustments as per treating team's judgement. Outcome Measures: Primary outcome was reduction in in-hospital mortality. Secondary outcomes were improvement in qSOFA score, pO2/fiO2 ratio, fall in inflammatory markers, need for mechanical ventilation and vasopressors. Results: Regarding primary outcome, 10 (33.3%) patients died in intervention group compared to 13 (43.3%) in placebo. Worth noting from baseline characteristics is that 86.7% in intervention arm were of severe category compared to 66.7% severe category patients in placebo group. Though number of severe cases were more in intervention arm there has been comparatively less mortality in this group. Regarding secondary outcomes, amongst 30 patients in vitamin C group, 11 (36.7%) required invasive mechanical ventilation compared to 14 (46.7%) out of 30 in placebo group but the difference was not statistically significant. Although there were a greater number of moderate cases in placebo group, invasive ventilation requirement (and NIV requirement) was more in this group, thus it could be considered that vitamin C might have a role in reducing the severity of disease. The need for vasopressor therapy was higher in intervention arm 33.3% compared to 26.7% in placebo but not significant statistically. The secondary outcomes of the study such as improvement in organ failure score (qSOFA Score), fall in level of inflammatory markers, improvement in respiratory index (pO2/fiO2 ratio), need for mechanical ventilation and need for vasopressors also shown encouraging results but not up to the statistically significant level due to moderate..
Financial support and sponsorship
Nil.
Conflicts of interest There are no conflicts of interest.
References
Abobaker, Alzwi, Alraied, Overview of the possible role of vitamin C in management of COVID-19, Pharmacol Rep
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-Final report, N Engl J Med
Fowler Aa 3 Rd, Truwit, Hite, Morris, Dewilde et al., Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI Randomized Clinical Trial, JAMA
Gao, Xu, Wang, Lv, Ma et al., The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study, Aging
Gordon, Angus, Derde, Interleukin-6 receptor antagonists in critically Ill patients with Covid-19. Reply, N Engl J Med
Grooth, Manubulu-Choo, Zandvliet, Spoelstra-De Man, Girbes et al., Vitamin C pharmacokinetics in critically Ill patients: A randomized trial of four IV regimens, Chest
Hiedra, Lo, Elbashabsheh, Gul, Wright et al., The use of IV vitamin C for patients with COVID-19: A case series, Expert Rev Anti Infect Ther
Hoang, Shaw, Fang, Han, Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection, J Glob Antimicrob Resist
Hu, Huang, Yin, The cytokine storm and COVID-19, J Med Virol
Jamalimoghadamsiahkali, Zarezade, Koolaji, Seyedalinaghi, Zendehdel et al., Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial, Eur J Med Res
Khan, Misdary, Yegya-Raman, Kim, Narayanan et al., Montelukast in hospitalized patients diagnosed with COVID-19, J Asthma, doi:10.1080/02770903.2021.1881967
Liu, Zhu, Zhang, Li, Peng, Intravenous high-dose vitamin C for the treatment of severe COVID-19: Study protocol for a multicentre randomised controlled trial, BMJ Open
O V I D - ; Sterne, Murthy, Diaz, Slutsky, Villar, Association between administration of systemic corticosteroids and mortality among critically Ill patients with covid-19: A meta-analysis, JAMA
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy, Repurposed antiviral drugs for Covid-19-Interim WHO solidarity trial results, N Engl J Med
Ravikirti, Pattadar, Raj, Agarwal, Biswas, Evaluation of ivermectin as a potential treatment for mild to moderate Covid-19: A double-blind randomized placebo controlled trial in eastern India, J Pharm Pharm Sci
Recovery Collaborative Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Wei, Wang, Liao, Guo, Wen et al., Efficacy of vitamin C in patients with sepsis: An updated meta-analysis, Eur J Pharmacol
Zhang, Rao, Li, Zhu, Liu et al., Pilot trial of high-dose vitamin C in critically ill COVID-19 patients, Ann Intensive Care
Zhu, Zhang, Li, Yang, Song, China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.4103/jfmpc.jfmpc_2437_21",
"ISSN": [
"2249-4863"
],
"URL": "http://dx.doi.org/10.4103/jfmpc.jfmpc_2437_21",
"alternative-id": [
"355454"
],
"author": [
{
"affiliation": [],
"family": "Kumar",
"given": "Vijay",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bhushan",
"given": "Divendu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Supriya",
"given": "Sushmita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ganapule",
"given": "AvinashAravind",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lohani",
"given": "Pallavi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shyama",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pandey",
"given": "Sanjay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Majhi",
"given": "PramodKumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anand",
"given": "Utpal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Ramesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhadani",
"given": "UmeshKumar",
"sequence": "additional"
}
],
"container-title": "Journal of Family Medicine and Primary Care",
"container-title-short": "J Family Med Prim Care",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
13
]
],
"date-time": "2022-09-13T10:27:00Z",
"timestamp": 1663064820000
},
"deposited": {
"date-parts": [
[
2022,
9,
13
]
],
"date-time": "2022-09-13T10:29:59Z",
"timestamp": 1663064999000
},
"indexed": {
"date-parts": [
[
2022,
9,
13
]
],
"date-time": "2022-09-13T11:17:57Z",
"timestamp": 1663067877480
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"member": "2581",
"original-title": [],
"page": "4758",
"prefix": "10.4103",
"published": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Medknow",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.4103/jfmpc.jfmpc_2437_21"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Materials Science"
],
"subtitle": [],
"title": "Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial",
"type": "journal-article",
"volume": "11"
}
