Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial
et al., Pharmaceuticals, doi:10.3390/ph15020200, NCT04912011, Feb 2022
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
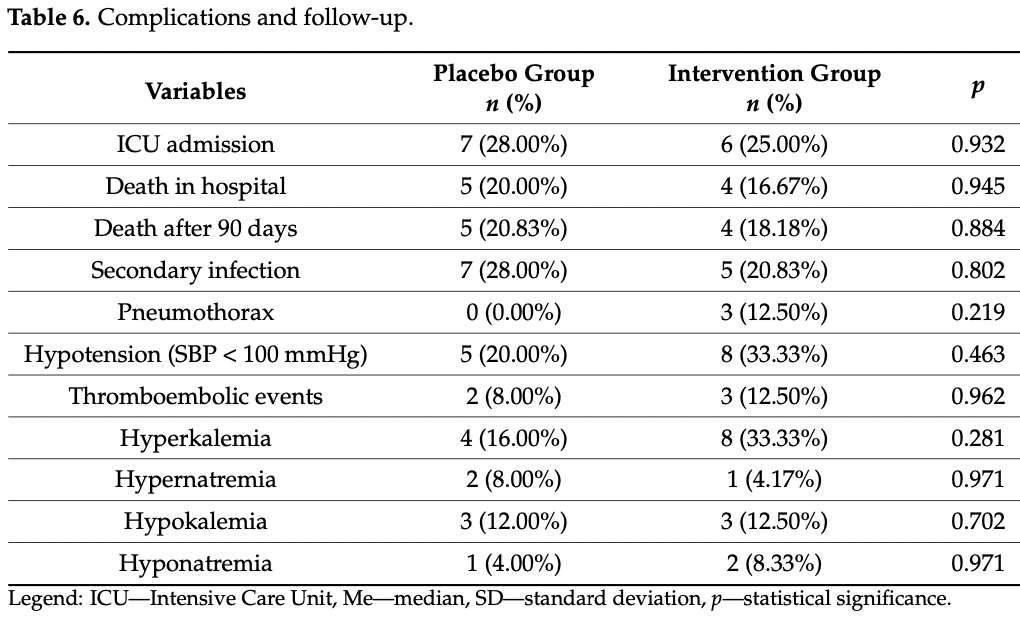
RCT with 24 patients treated with potassium canrenoate and 25 placebo patients in Poland, showing no significant differences.
|
risk of death, 16.7% lower, RR 0.83, p = 1.00, treatment 4 of 24 (16.7%), control 5 of 25 (20.0%), NNT 30.
|
|
risk of ICU admission, 10.7% lower, RR 0.89, p = 1.00, treatment 6 of 24 (25.0%), control 7 of 25 (28.0%), NNT 33.
|
|
relative TFS score, 30.4% better, RR 0.70, p = 0.51, treatment 24, control 25.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kotfis et al., 5 Feb 2022, Randomized Controlled Trial, placebo-controlled, Poland, peer-reviewed, 10 authors, study period December 2020 - August 2021, trial NCT04912011 (history).
Contact: katarzyna.kotfis@pum.edu.pl (corresponding author), igor.karolak@gmail.com, kacper.lechowicz@gmail.com, mazegan@wp.pl, apikulska@wp.pl, paulina.niedzwiedzka-rystwej@usz.edu.pl, kawamilosz@gmail.com, jsien@poczta.onet.pl, aleksandra.szylinska@gmail.com, mwisniewska35@gmail.com.
Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial
Pharmaceuticals, doi:10.3390/ph15020200
In December 2019 the SARS-CoV-2 virus appeared in the world, mainly presenting as an acute infection of the lower respiratory tract, namely pneumonia. Nearly 10% of all patients show significant pulmonary fibrotic changes after the infection. The aim of this study was to evaluate the effectiveness and safety of potassium canrenoate in the treatment of COVID-19-associated pneumonia and pulmonary fibrosis. We performed a randomized clinical trial (RCT) of potassium canrenoate vs placebo. A total of 55 patients were randomized and 49 were included in the final analysis (24 allocated to the intervention group and 25 allocated to the control group). Patients were assessed by physical examination, lung ultrasound, CT imaging and blood samples that underwent biochemical analysis. This RCT has shown that the administration of potassium canrenoate to patients with COVID-19 induced pneumonia was not associated with shorter mechanical ventilation time, shorter passive oxygenation, shorter length of hospitalization or less fibrotic changes on CT imaging. The overall mortality rate was not significantly different between the two groups. Adverse events recorded in this study were not significantly increased by the administration of potassium canrenoate. The negative outcome of the study may be associated with the relatively small number of patients included. Any possible benefits from the use of potassium canrenoate as an antifibrotic drug in COVID-19 patients require further investigation.
Author Contributions: Conceptualization, K.K. and J.S.; Data curation, I.K., M.K. and A.S.; Formal analysis, K.K., I.K., K.L., P.N.-R., M.K. and A.S.; Funding acquisition, K.K. and M.W.; Investigation, K.K., I.K., M.Z.-B. and M.K.; Methodology, K.K., I.K., K.L., A.P., P.N.-R., J.S., A.S. and M.W.; Project administration, K.K. and I.K.; Resources, K.K.; Software, I.K. and K.L.; Supervision, K.K. and A.S.; Validation, M.Z.-B., A.P., P.N.-R. and M.W.; Visualization, I.K. and A.S.; Writing-original draft, K.K., I.K. and A.S.; Writing-review and editing, K.L., M.Z.-B., A.P., P.N.-R., M.K., J.S. and M.W. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
References
Adams, Icheva, Deppisch, Lauer, Herrmann et al., Long-Term Pulmonal Therapy of Cystic Fibrosis-Patients with Amitriptyline, Cell. Physiol. Biochem, doi:10.1159/000445648
Alhiyari, Ata, Islam Alghizzawi, Bint I Bilal, Salih Abdulhadi et al., Post COVID-19 fibrosis, an emerging complicationof SARS-CoV-2 infection, IDCases, doi:10.1016/j.idcr.2020.e01041
Atalay, Dogan, Aykan, Gundogdu, Keles, The efficacy of spironolactone in the treatment of acute respiratory distress syndrome-induced rats, Singap. Med. J
Barut, Ozacmak, Turan, Sayan-Ozacmak, Aktunc, Reduction of Acute Lung Injury by Administration of Spironolactone After Intestinal Ischemia and Reperfusion in Rats, Clin. Invest. Med, doi:10.25011/cim.v39i1.26326
Cannavo, Bencivenga, Liccardo, Elia, Marzano et al., Aldosterone and Mineralocorticoid Receptor System in Cardiovascular Physiology and Pathophysiology, Oxid. Med. Cell. Longev, doi:10.1155/2018/1204598
Carpinteiro, Edwards, Hoffmann, Kochs, Gripp et al., Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells, Cell Rep. Med, doi:10.1016/j.xcrm.2020.100142
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study, Lancet
Combet, Pavot, Savale, Humbert, Monnet, Rapid onset honeycombing fibrosis in spontaneously breathing patient with COVID-19, Eur. Respir. J, doi:10.1183/13993003.01808-2020
Crosby, Waters, Epithelial repair mechanisms in the lung, Am. J. Physiol.-Lung Cell. Mol. Physiol, doi:10.1152/ajplung.00361.2009
Edwards, Klekot, Halugan, Korchev, Follow Your Nose: A Key Clue to Understanding and Treating COVID-19, Front. Endocrinol, doi:10.3389/fendo.2021.747744
Fraser, St Noble, Hoyles, Benamore, Ho, Readily accessible CT scoring method to quantify fibrosis in IPF, BMJ Open Respir. Res, doi:10.1136/bmjresp-2020-000584
Huang, Huang, Wang, Li, Ren et al., 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study, Lancet, doi:10.1016/S0140-6736(20)32656-8
Hui, Wong, Ko, Tam, Chan et al., The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors, Chest, doi:10.1378/chest.128.4.2247
Jeon, Son, Choi, Effect of Spironolactone on COVID-19 in Patients With Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea, Front. Med, doi:10.3389/fmed.2021.629176
Ji, Ma, Zhou, Zhang, Lu et al., Spironolactone attenuates bleomycin-induced pulmonary injury partially via modulating mononuclear phagocyte phenotype switching in circulating and alveolar compartments, PLoS ONE, doi:10.1371/journal.pone.0081090
Jover, Matilla, Garaikoetxea, Fernández-Celis, Muntendam et al., Beneficial effects of mineralocorticoid receptor pathway blockade against endothelial inflammation induced by sars-COV-2 spike protein, Biomedicines, doi:10.3390/biomedicines9060639
Khalifa, Yosri, El-Mallah, Ghonaim, Guo et al., Screening for natural and derived bio-active compounds in preclinical and clinical studies: One of the frontlines of fighting the coronaviruses pandemic, Phytomedicine, doi:10.1016/j.phymed.2020.153311
Kotfis, Lechowicz, Dro Żd Żal, Niedźwiedzka-Rystwej, Wojdacz et al., COVID-19-The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection, Pharmaceuticals, doi:10.3390/ph14010071
Kotfis, Williams Roberson, Wilson, Pun, Ely et al., COVID-19: What do we need to know about ICU delirium during the SARS-CoV-2 pandemic?, Anaesthesiol. Intensive Ther, doi:10.5114/ait.2020.95164
Kucewicz-Czech, Damps, Triage during the COVID-19 pandemic, Anaesthesiol. Intensive Ther, doi:10.5114/ait.2020.100564
Kumar, Zuo, Yalavarthi, Hunker, Knight et al., SARS-CoV-2 Spike Protein S1-Mediated Endothelial Injury and Pro-Inflammatory State Is Amplified by Dihydrotestosterone and Prevented by Mineralocorticoid Antagonism, Viruses, doi:10.3390/v13112209
Lechowicz, Dro Żd Żal, Machaj, Rosik, Szostak, COVID-19: The potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection, J. Clin. Med, doi:10.3390/jcm9061917
Lian, Jin, Hao, Jia, Cai et al., Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID-19) from Zhejiang province in China. Influenza Other, Respi. Viruses, doi:10.1111/irv.12758
Lieber, Fernandez, Mingo, Jia, Caniga et al., Mineralocorticoid receptor antagonists attenuate pulmonary inflammation and bleomycin-evoked fibrosis in rodent models, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2013.08.019
Loas, Le Corre, Update on Functional Inhibitors of Acid Sphingomyelinase (FIASMAs) in SARS-CoV-2 Infection, Pharmaceuticals, doi:10.3390/ph14070691
Maleszka, Kruszewski, Comparative evaluation of inhaling a single dose of furosemide or spironolactone on bronchial hyperreactivity of patients with atopic bronchial asthma, Pol. Tyg. Lek
Manivel, Lesnewski, Shamim, Carbonatto, Govindan, CLUE: COVID-19 lung ultrasound in emergency department, EMA-Emerg. Med. Australas, doi:10.1111/1742-6723.13546
Miller, Bruen, Schnaus, Zhang, Ali et al., Auxora versus standard of care for the treatment of severe or critical COVID-19 pneumonia: Results from a randomized controlled trial, Crit. Care, doi:10.1186/s13054-020-03220-x
Ngai, Ko, Ng, To, Tong et al., The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status, Respirology, doi:10.1111/j.1440-1843.2010.01720.x
Soldati, Smargiassi, Inchingolo, Buonsenso, Perrone et al., Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19, J. Ultrasound Med, doi:10.1002/jum.15285
Thille, Esteban, Fernández-Segoviano, Rodriguez, Aramburu et al., Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: A prospective cohort study of clinical autopsies, Lancet. Respir. Med, doi:10.1016/S2213-2600(13)70053-5
Umemura, Mitsuyama, Minami, Nishida, Watanabe et al., Efficacy and safety of nintedanib for pulmonary fibrosis in severe pneumonia induced by COVID-19: An interventional study, Int. J. Infect. Dis, doi:10.1016/j.ijid.2021.05.055
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA
Who Team, Summary of Probable SARS Cases with Onset of Illness from 1
Wilcox, Pitt, Is Spironolactone the Preferred Renin-Angiotensin-Aldosterone Inhibitor for Protection Against COVID-19?, J. Cardiovasc. Pharmacol, doi:10.1097/FJC.0000000000000960
Wu, Liu, Zhou, Yu, Li et al., 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00174-0
Wynn, Integrating mechanisms of pulmonary fibrosis, J. Exp. Med, doi:10.1084/jem.20110551
Xu, Zhang, Dai, IL-25/IL-33/TSLP contributes to idiopathic pulmonary fibrosis: Do alveolar epithelial cells and (myo)fibroblasts matter?, Exp. Biol. Med, doi:10.1177/1535370220915428
Yavas, Yavas, Celik, Sen, Ata et al., The impact of spironolactone on the lung injury induced by concomitant trastuzumab and thoracic radiotherapy, Int. J. Radiat. Res, doi:10.1016/j.jtho.2018.08.997
Yu, Liu, Xu, Zhang, Lan et al., Prediction of the development of pulmonary fibrosis using serial thin-section ct and clinical features in patients discharged after treatment for COVID-19 pneumonia, Korean J. Radiol
Zaafan, Haridy, Abdelhamid, Amitriptyline attenuates bleomycin-induced pulmonary fibrosis: Modulation of the expression of NF-κβ, iNOS, and Nrf2. Naunyn-Schmiedebergs, Arch. Pharmacol, doi:10.1007/s00210-018-1586-1
Zannad, Alla, Dousset, Perez, Pitt, Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: Insights from the randomized aldactone evaluation study (RALES). Rales Investigators, Circulation, doi:10.1161/01.CIR.102.22.2700
Zhang, Li, Liu, Han, Ju et al., Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: A 15-year follow-up from a prospective cohort study, Bone Res, doi:10.1038/s41413-020-0084-5
DOI record:
{
"DOI": "10.3390/ph15020200",
"ISSN": [
"1424-8247"
],
"URL": "http://dx.doi.org/10.3390/ph15020200",
"abstract": "<jats:p>In December 2019 the SARS-CoV-2 virus appeared in the world, mainly presenting as an acute infection of the lower respiratory tract, namely pneumonia. Nearly 10% of all patients show significant pulmonary fibrotic changes after the infection. The aim of this study was to evaluate the effectiveness and safety of potassium canrenoate in the treatment of COVID-19-associated pneumonia and pulmonary fibrosis. We performed a randomized clinical trial (RCT) of potassium canrenoate vs placebo. A total of 55 patients were randomized and 49 were included in the final analysis (24 allocated to the intervention group and 25 allocated to the control group). Patients were assessed by physical examination, lung ultrasound, CT imaging and blood samples that underwent biochemical analysis. This RCT has shown that the administration of potassium canrenoate to patients with COVID-19 induced pneumonia was not associated with shorter mechanical ventilation time, shorter passive oxygenation, shorter length of hospitalization or less fibrotic changes on CT imaging. The overall mortality rate was not significantly different between the two groups. Adverse events recorded in this study were not significantly increased by the administration of potassium canrenoate. The negative outcome of the study may be associated with the relatively small number of patients included. Any possible benefits from the use of potassium canrenoate as an antifibrotic drug in COVID-19 patients require further investigation.</jats:p>",
"alternative-id": [
"ph15020200"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8430-1369",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kotfis",
"given": "Katarzyna",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8049-0833",
"affiliation": [],
"authenticated-orcid": false,
"family": "Karolak",
"given": "Igor",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0807-8510",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lechowicz",
"given": "Kacper",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6532-9649",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zegan-Barańska",
"given": "Małgorzata",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pikulska",
"given": "Agnieszka",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4065-3842",
"affiliation": [],
"authenticated-orcid": false,
"family": "Niedźwiedzka-Rystwej",
"given": "Paulina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kawa",
"given": "Miłosz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sieńko",
"given": "Jerzy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6105-5329",
"affiliation": [],
"authenticated-orcid": false,
"family": "Szylińska",
"given": "Aleksandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wiśniewska",
"given": "Magda",
"sequence": "additional"
}
],
"container-title": [
"Pharmaceuticals"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T01:38:40Z",
"timestamp": 1644197920000
},
"deposited": {
"date-parts": [
[
2022,
2,
14
]
],
"date-time": "2022-02-14T09:46:40Z",
"timestamp": 1644832000000
},
"indexed": {
"date-parts": [
[
2022,
4,
3
]
],
"date-time": "2022-04-03T10:54:11Z",
"timestamp": 1648983251903
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1424-8247"
}
],
"issue": "2",
"issued": {
"date-parts": [
[
2022,
2,
5
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2022,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
5
]
],
"date-time": "2022-02-05T00:00:00Z",
"timestamp": 1644019200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1424-8247/15/2/200/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "200",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
2,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
5
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.5114/ait.2020.100564",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1111/irv.12758",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.5114/ait.2020.95164",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/S0140-6736(20)32656-8",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1378/chest.128.4.2247",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1111/j.1440-1843.2010.01720.x",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"key": "ref9",
"unstructured": "World Health Organization WHO Coronavirus (COVID-19) Dashboardhttps://covid19.who.int"
},
{
"DOI": "10.3390/jcm9061917",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/S2213-2600(21)00174-0",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"key": "ref12",
"series-title": "Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003",
"year": "2015"
},
{
"DOI": "10.1038/s41413-020-0084-5",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.25011/cim.v39i1.26326",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.jtho.2018.08.997",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1161/01.CIR.102.22.2700",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.3390/v13112209",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.3389/fendo.2021.747744",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1155/2018/1204598",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.phymed.2020.153311",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.ejphar.2013.08.019",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1371/journal.pone.0081090",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.3390/ph14010071",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"article-title": "The efficacy of spironolactone in the treatment of acute respiratory distress syndrome-induced rats",
"author": "Atalay",
"first-page": "501",
"journal-title": "Singap. Med. J.",
"key": "ref24",
"volume": "51",
"year": "2010"
},
{
"article-title": "Comparative evaluation of inhaling a single dose of furosemide or spironolactone on bronchial hyperreactivity of patients with atopic bronchial asthma",
"author": "Maleszka",
"first-page": "415",
"journal-title": "Pol. Tyg. Lek.",
"key": "ref25",
"volume": "49",
"year": "1994"
},
{
"DOI": "10.1007/s00210-018-1586-1",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.3390/ph14070691",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1016/j.xcrm.2020.100142",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1159/000445648",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1097/FJC.0000000000000960",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3348/kjr.2020.0215",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1084/jem.20110551",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1177/1535370220915428",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1152/ajplung.00361.2009",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.3390/biomedicines9060639",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.ijid.2021.05.055",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1183/13993003.01808-2020",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1016/j.idcr.2020.e01041",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1186/s13054-020-03220-x",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.3389/fmed.2021.629176",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/S2213-2600(13)70053-5",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1002/jum.15285",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1111/1742-6723.13546",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1136/bmjresp-2020-000584",
"doi-asserted-by": "publisher",
"key": "ref44"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1424-8247/15/2/200"
}
},
"score": 1,
"short-container-title": [
"Pharmaceuticals"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Drug Discovery",
"Pharmaceutical Science",
"Molecular Medicine"
],
"subtitle": [],
"title": [
"Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial"
],
"type": "journal-article",
"volume": "15"
}