Androgen deprivation and SARS-CoV-2 in men with prostate cancer
et al., Annals of Oncology, doi:10.1016/j.annonc.2020.06.015, Jun 2020
7th treatment shown to reduce risk in
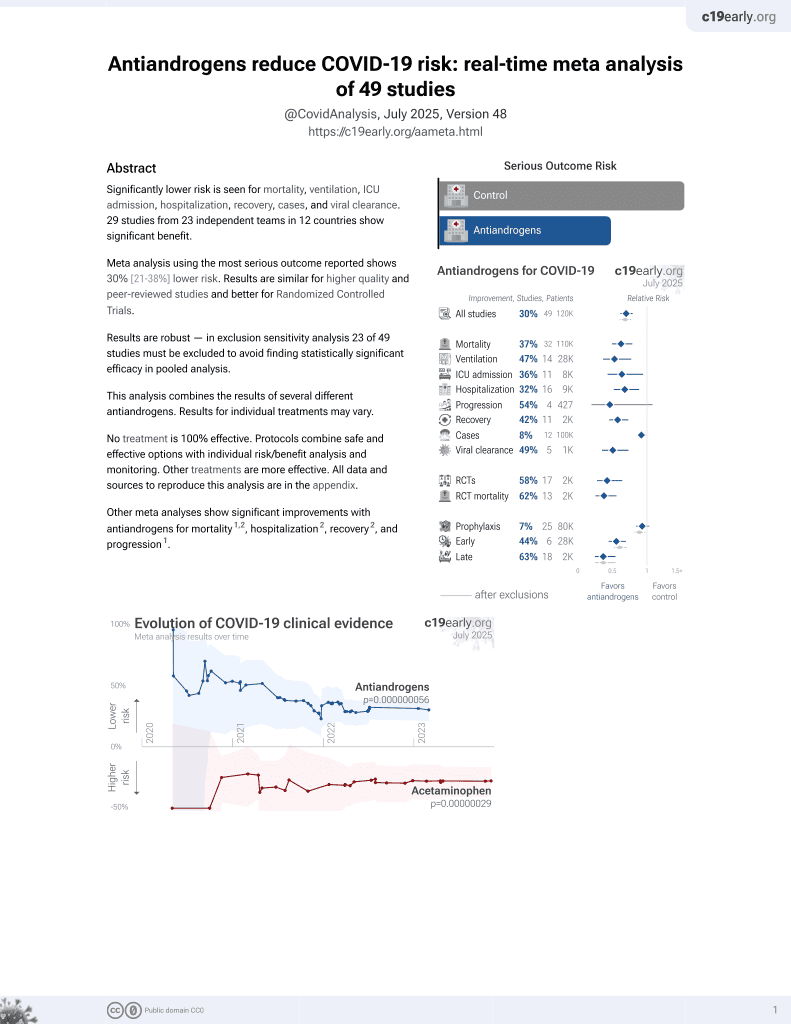
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 352 prostate cancer patients in Finland, showing no significant differences in COVID-19 with ADT.
Although the 46% lower mortality is not statistically significant, it is consistent with the significant 37% lower mortality [21‑50%] from meta-analysis of the 32 mortality results to date.
|
risk of death, 45.8% lower, RR 0.54, p = 1.00, treatment 1 of 134 (0.7%), control 3 of 218 (1.4%), NNT 159.
|
|
risk of death/ICU, 45.8% lower, RR 0.54, p = 1.00, treatment 1 of 134 (0.7%), control 3 of 218 (1.4%), NNT 159.
|
|
risk of case, 11.3% lower, RR 0.89, p = 1.00, treatment 6 of 134 (4.5%), control 11 of 218 (5.0%), NNT 176.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Koskinen et al., 29 Jun 2020, retrospective, Finland, peer-reviewed, 7 authors.
Abstract: Annals of Oncology
Letters to the Editor
Androgen deprivation and SARS-CoV-2 in men
with prostate cancer
We read with great interest the very recent article by
Montopoli et al.,1 which reports men with prostate cancer
tested for severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) in Veneto, Italy, by 1 April 2020. The authors
suggest that androgen deprivation therapy (ADT) could
partially protect from SARS-CoV-2 infection.1 The biological
premise for this observation is the androgen receptormediated regulation of TMPRSS2,2 a type II transmembrane serine protease that is important for SARS-CoV-2
entry to host cells.3 Indeed, androgens regulate TMPRSS2
expression also in a lung carcinoma cell line.4
Encouraged by the findings of Montopoli et al.,1 we
examined the health care records of patients with prostate
cancer [International Classification of Diseases (ICD)-10
code C61] in the Hospital District of Helsinki and Uusimaa,
Finland, using automated text mining with manual verification and structured diagnostic codes. Altogether, 352
such men were tested for SARS-CoV-2 between 7 March
and 14 May 2020. A patient was classified to be on ADT if
he had a history of orchiectomy, or a valid prescription for
a gonadotropin-releasing hormone (GnRH) analogue, GnRH
antagonist, and/or antiandrogens (flutamide, bicalutamide,
enzalutamide) or the CYP17 inhibitor abiraterone before
his SARS-CoV-2 test (n ¼ 134) [38%, 95% confidence interval (CI): 33%e43%]. The mean age of these 134 men
was 78.4 years 8.1 standard deviation (range 58e96
years). The frequency of being on ADT was in agreement
with that observed in a survey of a large cohort of UK men
with prostate cancer.5 Conversely, a patient was classified
not to be on ADT if no records of the above conditions
were found or ADT had been ceased before a SARS-CoV-2
test (n ¼ 218; mean age 76.5 years 9.4 standard deviation, range 51e96 years). The presence of SARS-CoV-2
RNA in nasopharyngeal swab samples was analyzed by
RT-PCR (details available upon request). This study was
based on register data, provided by the registry holder,
Helsinki University Hospital, and therefore no ethical
permission was required according to the Finnish Medical
Research Act.
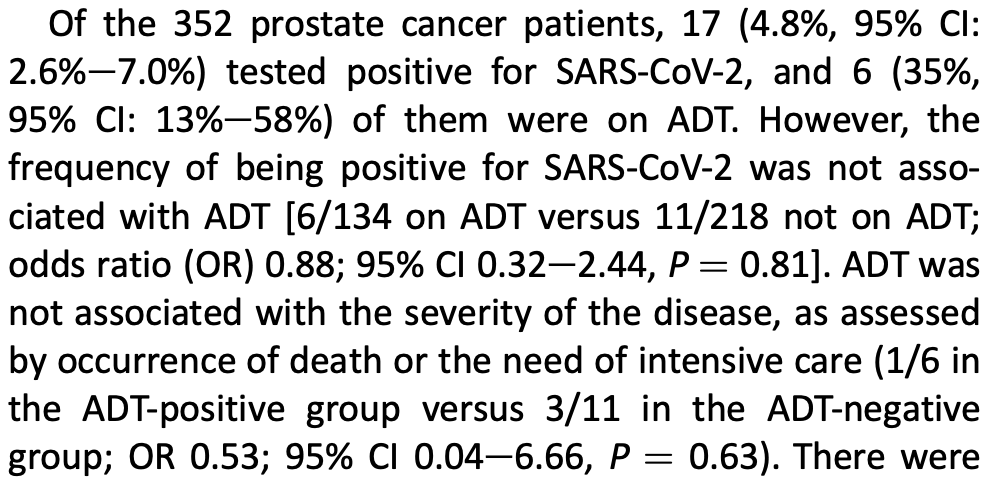
Of the 352 prostate cancer patients, 17 (4.8%, 95% CI:
2.6%e7.0%) tested positive for SARS-CoV-2, and 6 (35%,
95% CI: 13%e58%) of them were on ADT. However, the
frequency of being positive for SARS-CoV-2 was not associated with ADT [6/134 on ADT versus 11/218 not on ADT;
odds ratio (OR) 0.88; 95% CI 0.32e2.44, P ¼ 0.81]. ADT was
not associated with the severity of the disease, as assessed
by occurrence of death or the need of intensive care (1/6 in
the ADT-positive group versus 3/11 in the ADT-negative
group; OR 0.53; 95% CI 0.04e6.66, P ¼ 0.63). There were
no differences in possible confounding comorbidities on
Volume 31 - Issue 10 - 2020
Table 1. The presence of potential confounding factors on COVID-19
severity in SARS-CoV-2 tested patients with prostate cancer classified on
the basis of being on androgen deprivation therapy
Age > 65 years
Hypertensionb
Coronary artery diseasec
COPDd
Diabetese
Cardiac arrhythmiaf
Current smoker
No ADT
(n [ 218)
On ADT
(n [ 134)
P valuea
191
47
30
12
17
41
17
125
30
21
8
16
30
18
0.10
0.89
0.64
1.0
0.26
0.42
0.10
The distributions of diagnoses from 2015 to 2 weeks before the SARS-CoV-2 test are
shown. Smoking status was extracted by using automated text mining and manual
verification. The data denote the..
DOI record:
{
"DOI": "10.1016/j.annonc.2020.06.015",
"ISSN": [
"0923-7534"
],
"URL": "http://dx.doi.org/10.1016/j.annonc.2020.06.015",
"alternative-id": [
"S0923753420399257"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Androgen deprivation and SARS-CoV-2 in men with prostate cancer"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Annals of Oncology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.annonc.2020.06.015"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Published by Elsevier Ltd on behalf of European Society for Medical Oncology."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7267-5811",
"affiliation": [],
"authenticated-orcid": false,
"family": "Koskinen",
"given": "M.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7847-5706",
"affiliation": [],
"authenticated-orcid": false,
"family": "Carpen",
"given": "O.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3428-4307",
"affiliation": [],
"authenticated-orcid": false,
"family": "Honkanen",
"given": "V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9733-3650",
"affiliation": [],
"authenticated-orcid": false,
"family": "Seppänen",
"given": "M.R.J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5184-9616",
"affiliation": [],
"authenticated-orcid": false,
"family": "Miettinen",
"given": "P.J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2727-8849",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tuominen",
"given": "J.A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5385-434X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Raivio",
"given": "T.",
"sequence": "additional"
}
],
"container-title": [
"Annals of Oncology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"annalsofoncology.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
6,
29
]
],
"date-time": "2020-06-29T15:35:22Z",
"timestamp": 1593444922000
},
"deposited": {
"date-parts": [
[
2021,
10,
5
]
],
"date-time": "2021-10-05T07:13:11Z",
"timestamp": 1633417991000
},
"funder": [
{
"name": "Juha Vainio Foundation"
}
],
"indexed": {
"date-parts": [
[
2022,
2,
26
]
],
"date-time": "2022-02-26T03:49:18Z",
"timestamp": 1645847358752
},
"is-referenced-by-count": 13,
"issn-type": [
{
"type": "print",
"value": "0923-7534"
}
],
"issue": "10",
"issued": {
"date-parts": [
[
2020,
10
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2020,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "http://www.elsevier.com/open-access/userlicense/1.0/",
"content-version": "vor",
"delay-in-days": 365,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0923753420399257?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0923753420399257?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1417-1418",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532)",
"author": "Montopoli",
"doi-asserted-by": "crossref",
"first-page": "1040",
"issue": "8",
"journal-title": "Ann Oncol",
"key": "10.1016/j.annonc.2020.06.015_bib1",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1158/0008-5472.CAN-06-3332",
"article-title": "Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer",
"author": "Mostaghel",
"doi-asserted-by": "crossref",
"first-page": "5033",
"journal-title": "Cancer Res",
"key": "10.1016/j.annonc.2020.06.015_bib2",
"volume": "67",
"year": "2007"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.annonc.2020.06.015_bib3",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.mce.2009.12.022",
"article-title": "Androgen receptor and androgen-dependent gene expression in lung",
"author": "Mikkonen",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Mol Cell Endocrinol",
"key": "10.1016/j.annonc.2020.06.015_bib4",
"volume": "317",
"year": "2010"
},
{
"DOI": "10.1016/j.canep.2019.04.006",
"article-title": "Key factors associated with social distress after prostate cancer: results from the United Kingdom life after prostate cancer diagnosis study",
"author": "Wright",
"doi-asserted-by": "crossref",
"first-page": "201",
"journal-title": "Cancer Epidemiol",
"key": "10.1016/j.annonc.2020.06.015_bib5",
"volume": "60",
"year": "2019"
},
{
"article-title": "Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis",
"author": "Zheng",
"journal-title": "J Infect",
"key": "10.1016/j.annonc.2020.06.015_bib6",
"year": "2020"
}
],
"reference-count": 6,
"references-count": 6,
"relation": {},
"score": 1,
"short-container-title": [
"Annals of Oncology"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Oncology",
"Hematology"
],
"subtitle": [],
"title": [
"Androgen deprivation and SARS-CoV-2 in men with prostate cancer"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "31"
}