People exposed to proton‐pump inhibitors shortly preceding COVID‐19 diagnosis are not at an increased risk of subsequent hospitalizations and mortality: A nationwide matched cohort study
et al., British Journal of Clinical Pharmacology, doi:10.1111/bcp.15525, Oct 2022
PPIs for COVID-19
1st treatment shown to increase risk in
September 2020, now with p = 0.000000048 from 40 studies.
6,400+ studies for
210+ treatments. c19early.org
|
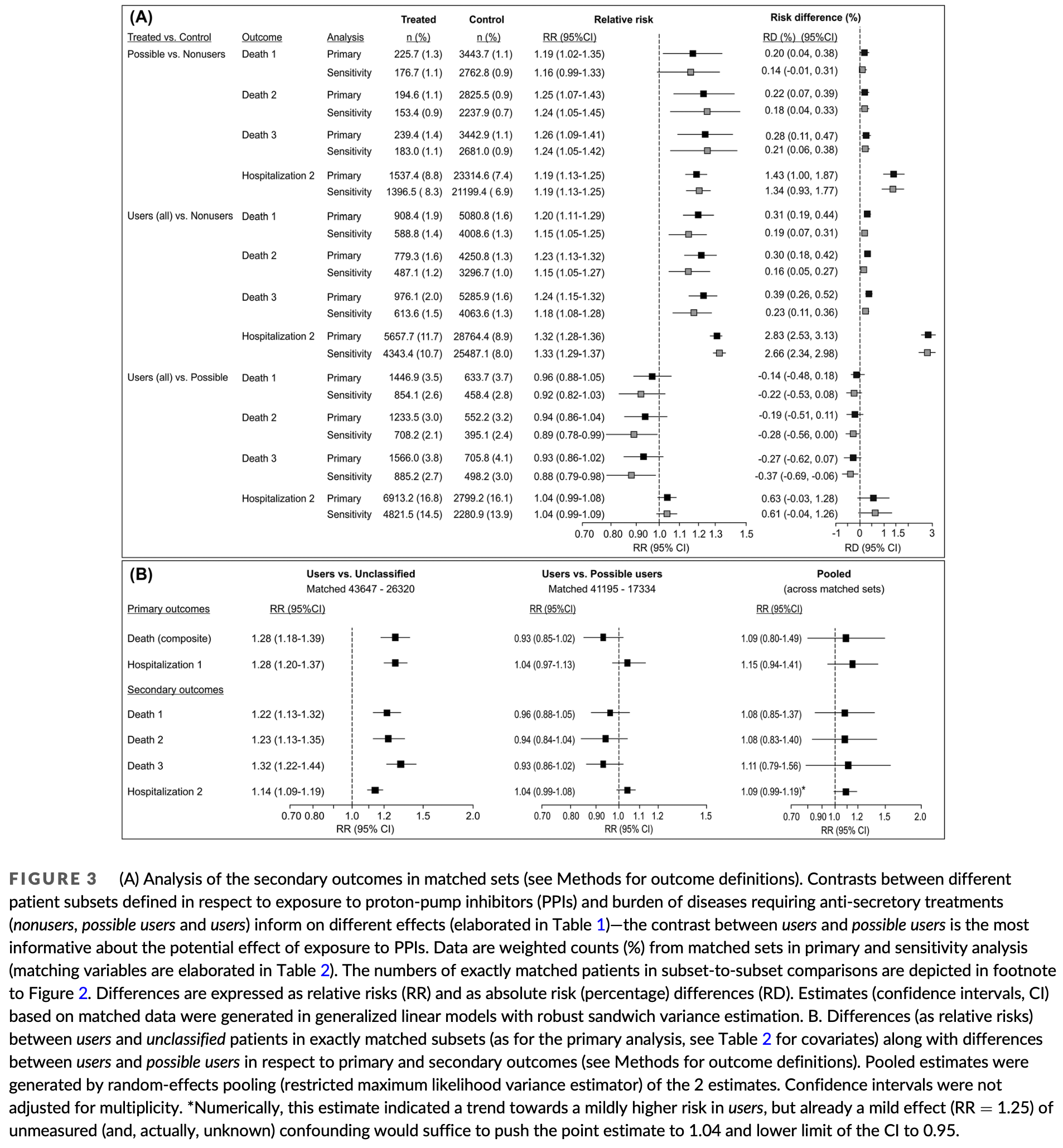
Retrospective 433,609 COVID-19 patients in Croatia showing no significant difference in mortality or hospitalization risk with proton-pump inhibitor (PPI) use before COVID-19 diagnosis compared to matched controls with PPI-requiring morbidities but no PPI prescriptions. There was significantly higher hospitalization for users with 1-3 prescriptions which authors do not comment on.
The classification of users and possible users may introduce confounding. Users required a PPI prescription, while possible users includes those with ≥3 NSAID prescriptions. Possible users may be OTC PPI users, and may differ significantly in NSAID use. NSAID use per group is not reported, and was not used in adjustments.
|
risk of death, 7.0% lower, RR 0.93, p = 0.12, treatment 41,195, control 17,334.
|
|
risk of hospitalization, 4.0% higher, OR 1.04, p = 0.32, treatment 41,195, control 17,334, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kodvanj et al., 5 Oct 2022, retrospective, Croatia, peer-reviewed, 3 authors, study period 25 February, 2020 - 15 August, 2021.
Contact: vladimir.trkulja@mef.hr.
People exposed to proton‐pump inhibitors shortly preceding COVID‐19 diagnosis are not at an increased risk of subsequent hospitalizations and mortality: A nationwide matched cohort study
British Journal of Clinical Pharmacology, doi:10.1111/bcp.15525
Aims: To assess whether exposure to proton-pump inhibitors (PPIs) shortly preceding COVID-19 diagnosis affected the risk of subsequent hospitalizations and mortality.
Methods : This population-based study embraced first COVID-19 episodes in adults diagnosed up to 15 August 2021 in Croatia. Patients were classified based on exposure to PPIs and burden of PPI-requiring morbidities as nonusers (no issued prescriptions, no recorded treatment-requiring conditions between 1 January 2019 and COVID-19 diagnosis), possible users (no issued prescriptions, but morbidities present; self-medication possible) and users (≥1 prescription within 3 months prior to the COVID-19 diagnosis; morbidities present). Subsets were mutually exactly matched for pre-COVID-19 characteristics. The contrast between users and possible users informed about the effect of PPIs that is separate of the effect of PPI-requiring conditions. Results: Among 433 609 patients, users and possible users were matched 41 195 (of 55 098) to 17 334 (of 18 170) in the primary and 33 272 to 16 434 in the sensitivity analysis. There was no relevant difference between them regarding mortality (primary: relative risk [RR] = 0.93 [95% confidence interval 0.85-1.02; absolute risk difference [RD] = À0.34% [À0.73, 0.03]; sensitivity: RR = 0.88 [0.78-0.98]; RD = À0.45% [À0.80, À0.11]) or hospitalizations (primary: RR = 1.04 [0.97-1.13]; RD = 0.29% [À0.16, 0.73]; sensitivity: RR = 1.05 [0.97-1.15]; RD = 0.32% [À0.12, 0.75]). The risks of both were slightly higher in possible users or users than in nonusers (absolutely by $0.4-1.6%) indicating the effect of PPI-requiring morbidities. Conclusion: Premorbid exposure to PPIs does not affect the risk of death or hospitalization in adult COVID-19 patients, but PPI-requiring morbidities seemingly slightly increase the risk of both.
References
Charpiat, Bleyzac, Tod, Proton pump inhibitors are risk factors for viral infections: even for COVID-19?, Clin Drug Investig, doi:info:doi/10.1007/s40261-020-00963-x
Dharmarajan, The use and misuse of proton pump inhibitors: an opportunity for deprescribing, J Am Med Dir Assoc, doi:info:doi/10.1016/j.jamda.2020.09.046
Fan, Liu, Miyata, Effect of acid suppressants on the risk of COVID-19: a propensity score-matched study using UK biobank, Gastroenterology, doi:info:doi/10.1053/j.gastro.2020.09.028
Freedberg, Kim, Yang, The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association, Gastroenterology, doi:info:doi/10.1053/j.gastro.2017.01.031
Griffith, Morris, Tudball, Collider bias undermines our understanding of COVID-19 disease risk and severity, Nat Commun, doi:info:doi/10.1038/s41467-020-19478-2
Haastrup, Thompson, Søndergaard, Jarbøl, Side effects of long-term proton pump inhibitor use: a review, Basic Clin Pharmacol Toxicol, doi:info:doi/10.1111/bcpt.13023
Homolak, Kodvanj, Trkulja, An additional perspective on proton pump inhibitors as risk factors for COVID-19, Clin Drug Investig, doi:info:doi/10.1007/s40261-021-01007-8
Homolak, Kodvanj, Widely available lysosome targeting agents should be considered as potential therapy for COVID-19, Int J Antimicrob Agents, doi:info:doi/10.1016/j.ijantimicag.2020.106044
Israelsen, Ernst, Lundh, Proton pump inhibitor use is not strongly associated with SARS-CoV-2 related outcomes: a nationwide study and meta-analysis, Clin Gastroenterol Hepatol, doi:info:doi/10.1016/j.cgh.2021.05.011
Kim, Kim, Wolf, Acid suppressant use in association with incidence and severe outcomes of COVID-19: a systematic review and meta-analysis, Eur J Clin Pharmacol, doi:info:doi/10.1007/s00228-021-03255-1
King, Nielsen, Why propensity scores should not be used for matching, Polit Anal, doi:info:doi/10.1017/pan.2019.11
Lee, Ha, Yeniova, Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching, Gut, doi:info:doi/10.1136/gutjnl-2020-322248
Lee, Yang, Yoo, Proton pump inhibitors and the risk of severe COVID-19: a post-hoc analysis from the Korean nationwide cohort, Gut, doi:info:doi/10.1136/gutjnl-2020-323672
Pranata, Huang, Lawrensia, Proton pump inhibitor on susceptibility to COVID-19 and its severity: a systematic review and meta-analysis, Pharmacol Rep, doi:info:doi/10.1007/s43440-021-00263-x
Richter, Rubenstein, Presentation and epidemiology of gastroesophageal reflux disease, Gastroenterology, doi:info:doi/10.1053/j.gastro.2017.07.045
Salvo, Ferko, Cash, Gonzalez, Kahrilas, Umbrella review of 42 systematic reviews with meta-analyses: the safety of proton pump inhibitors, Aliment Pharmacol Ther, doi:info:doi/10.1111/apt.16407
Schubert, Proton pump inhibitors: misconceptions and proper prescribing practice, Curr Opin Gastroenterol, doi:info:doi/10.1097/MOG.0000000000000676
Tas ¸temur S ¸, Ata7, Is it possible to use proton pump inhibitors in COVID-19 treatment and prophylaxis?, Med Hypotheses, doi:info:doi/10.1016/j.mehy.2020.110018
Yan, Chen, Sun, Does proton pump inhibitor use lead to a higher risk of coronavirus disease 2019 infection and progression to severe disease? A meta-analysis, Jpn J Infect Dis, doi:info:doi/10.7883/yoken.JJID.2021.074
Yibirin, Oliveira, Valera, Plitt, Lutgen, Adverse effects associated with proton pump inhibitor use, Cureus, doi:info:doi/10.7759/cureus.12759
DOI record:
{
"DOI": "10.1111/bcp.15525",
"ISSN": [
"0306-5251",
"1365-2125"
],
"URL": "http://dx.doi.org/10.1111/bcp.15525",
"abstract": "<jats:sec><jats:title>Aims</jats:title><jats:p>To assess whether exposure to proton‐pump inhibitors (PPIs) shortly preceding COVID‐19 diagnosis affected the risk of subsequent hospitalizations and mortality.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This population‐based study embraced first COVID‐19 episodes in adults diagnosed up to 15 August 2021 in Croatia. Patients were classified based on exposure to PPIs and burden of PPI‐requiring morbidities as <jats:italic>nonusers</jats:italic> (no issued prescriptions, no recorded treatment‐requiring conditions between 1 January 2019 and COVID‐19 diagnosis), <jats:italic>possible users</jats:italic> (no issued prescriptions, but morbidities present; self‐medication possible) and <jats:italic>users</jats:italic> (≥1 prescription within 3 months prior to the COVID‐19 diagnosis; morbidities present). Subsets were mutually exactly matched for pre‐COVID‐19 characteristics. The contrast between <jats:italic>users</jats:italic> and <jats:italic>possible users</jats:italic> informed about the effect of PPIs that is separate of the effect of PPI‐requiring conditions.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among 433 609 patients, <jats:italic>users</jats:italic> and <jats:italic>possible users</jats:italic> were matched 41 195 (of 55 098) to 17 334 (of 18 170) in the primary and 33 272 to 16 434 in the sensitivity analysis. There was no relevant difference between them regarding mortality (primary: relative risk [RR] = 0.93 [95% confidence interval 0.85–1.02; absolute risk difference [RD] = −0.34% [−0.73, 0.03]; sensitivity: RR = 0.88 [0.78–0.98]; RD = −0.45% [−0.80, −0.11]) or hospitalizations (primary: RR = 1.04 [0.97–1.13]; RD = 0.29% [−0.16, 0.73]; sensitivity: RR = 1.05 [0.97–1.15]; RD = 0.32% [−0.12, 0.75]). The risks of both were slightly higher in <jats:italic>possible users</jats:italic> or <jats:italic>users</jats:italic> than in <jats:italic>nonusers</jats:italic> (absolutely by ~0.4–1.6%) indicating the effect of PPI‐requiring morbidities.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Premorbid exposure to PPIs does not affect the risk of death or hospitalization in adult COVID‐19 patients, but PPI‐requiring morbidities seemingly slightly increase the risk of both.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/bcp.15525"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1359-3701",
"affiliation": [
{
"name": "Department of Gastroenterology and Hepatology University Hospital Center Zagreb Zagreb Croatia"
}
],
"authenticated-orcid": false,
"family": "Kodvanj",
"given": "Ivan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pharmacology Zagreb University School of Medicine Zagreb Croatia"
}
],
"family": "Homolak",
"given": "Jan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0968-1194",
"affiliation": [
{
"name": "Department of Pharmacology Zagreb University School of Medicine Zagreb Croatia"
}
],
"authenticated-orcid": false,
"family": "Trkulja",
"given": "Vladimir",
"sequence": "additional"
}
],
"container-title": "British Journal of Clinical Pharmacology",
"container-title-short": "Brit J Clinical Pharma",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
12
]
],
"date-time": "2022-09-12T14:09:31Z",
"timestamp": 1662991771000
},
"deposited": {
"date-parts": [
[
2023,
8,
20
]
],
"date-time": "2023-08-20T08:43:38Z",
"timestamp": 1692521018000
},
"funder": [
{
"DOI": "10.13039/501100007682",
"doi-asserted-by": "publisher",
"name": "Medicinski Fakultet, Sveučilište u Zagrebu"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:54:19Z",
"timestamp": 1714524859024
},
"is-referenced-by-count": 1,
"issue": "2",
"issued": {
"date-parts": [
[
2022,
10,
5
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2023,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
5
]
],
"date-time": "2022-10-05T00:00:00Z",
"timestamp": 1664928000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/bcp.15525",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/bcp.15525",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/bcp.15525",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "787-831",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
10,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
5
]
]
},
"published-print": {
"date-parts": [
[
2023,
2
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1097/MOG.0000000000000676",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1053/j.gastro.2017.01.031",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1016/j.jamda.2020.09.046",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"key": "e_1_2_10_5_1",
"unstructured": "Association of the European Self‐Care Industry.AESGP OTC ingredients directory.https://aesgp.eu/databases"
},
{
"DOI": "10.1111/bcpt.13023",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.7759/cureus.12759",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1111/apt.16407",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1007/s40261‐020‐00963‐x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1007/s40261‐021‐01007‐8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106044",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1016/j.mehy.2020.110018",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1016/j.cgh.2021.05.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1007/s43440‐021‐00263‐x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.7883/yoken.JJID.2021.074",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1007/s00228‐021‐03255‐1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1038/s41467‐020‐19478‐2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1053/j.gastro.2020.09.028",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1136/gutjnl‐2020‐322248",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1136/gutjnl‐2020‐323672",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1017/pan.2019.11",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1053/j.gastro.2017.07.045",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"key": "e_1_2_10_23_1",
"unstructured": "Medicinal products database. Agency for Medicinal Products and Medical Devices of Croatia.https://www.halmed.hr/Lijekovi/Baza-lijekova/#rezultati"
},
{
"DOI": "10.18637/jss.v042.i08",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"key": "e_1_2_10_25_1",
"unstructured": "R Core Team.R: a language and environment for statistical computing. R Foundation for Statistical Computing Vienna Austria;2020."
},
{
"DOI": "10.7326/M16‐2607",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1136/ebmental‐2019‐300117",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"article-title": "The concise guide to pharmacology 2019/20: enzymes",
"author": "Alexander SPH",
"first-page": "S297",
"journal-title": "Br J Pharmacol",
"key": "e_1_2_10_28_1",
"volume": "176",
"year": "2019"
},
{
"DOI": "10.1177/003591576505800503",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1007/s10654‐019‐00494‐6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_1"
},
{
"DOI": "10.1093/aje/kwr352",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"author": "Pearl J",
"first-page": "417",
"key": "e_1_2_10_32_1",
"volume-title": "Proceedings of UAI",
"year": "2010"
},
{
"DOI": "10.5056/jnm21095",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_1"
},
{
"DOI": "10.1371/journal.pone.0264704",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_34_1"
},
{
"DOI": "10.1186/s12879‐022‐07262‐0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_35_1"
},
{
"key": "e_1_2_10_36_1",
"unstructured": "Croatia: coronavirus pandemic country profile. Our World in Data (Accessed July 2 2022)."
},
{
"DOI": "10.1097/01.mlr.0000182534.19832.83",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_1"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2022.04.30.22274526",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.15525"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "People exposed to proton‐pump inhibitors shortly preceding COVID‐19 diagnosis are not at an increased risk of subsequent hospitalizations and mortality: A nationwide matched cohort study",
"type": "journal-article",
"volume": "89"
}