A randomised-controlled Phase I de-escalation trial of Molnupiravir and Nirmatrelvir/Ritonavir combination for mild-moderate SARS-CoV-2 infection
et al., medRxiv, doi:10.1101/2025.05.01.25326797, AGILE CST-8, NCT04746183, May 2025
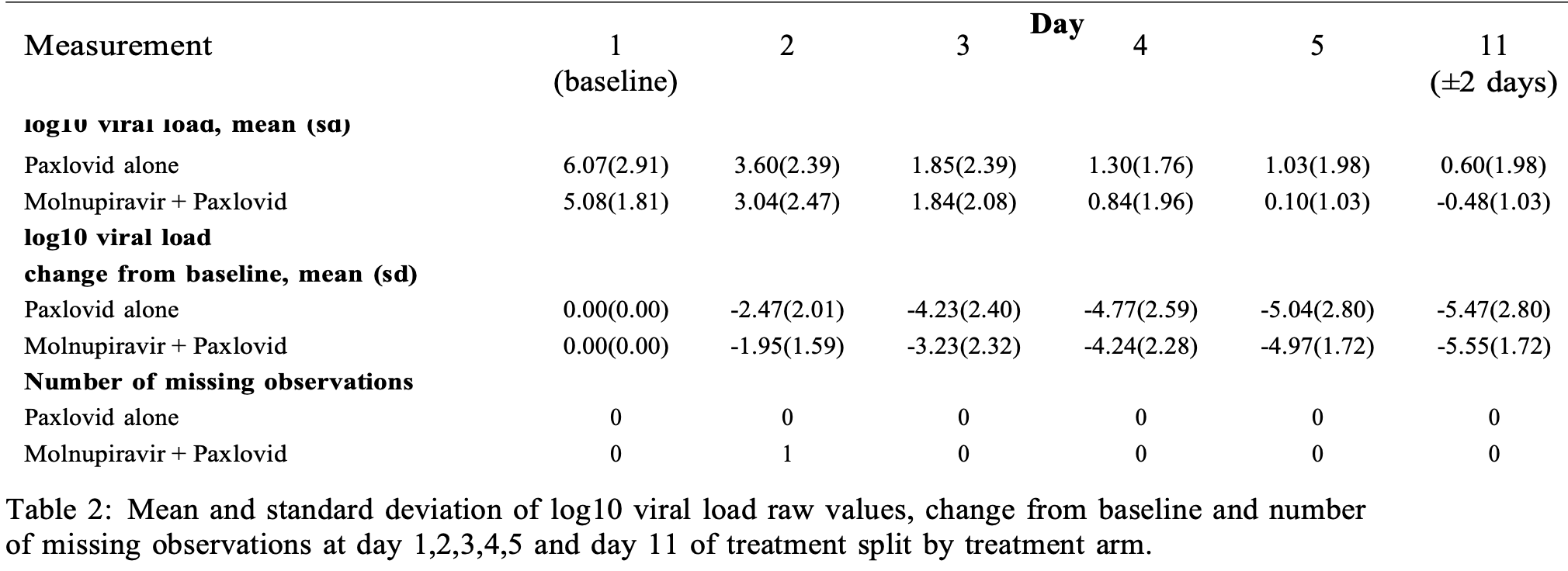
Randomized open-label phase I trial of 24 outpatients with mild-moderate COVID-19 showing safety and tolerability of combined molnupiravir and paxlovid therapy. Supplemental Table 2 shows greater decline of viral load in the control group for days 2-5. Treatment groups are not clear - the paper reports that the control group received standard of care and "no participant in the standard of care arm received any antiviral therapy", the CONSORT diagram indicates the control group received placebo, and the table in the supplementary data indicates the control group received paxlovid. Authors report faster initial viral clearance with treatment using a bi-exponential model, although this did not reach significance without the assumption that treatment effects only the "fast" phase and not the "persistent" phase, and results are limited with the small sample size and baseline differences.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers molnupiravir and paxlovid.
|
viral load, 1.4% higher, relative load 1.01, p = 0.94, treatment mean 4.97 (±1.72) n=16, control mean 5.04 (±2.8) n=8, relative reduction in viral load, mid-recovery, day 5.
|
|
viral load, 12.5% higher, relative load 1.12, p = 0.61, treatment mean 4.24 (±2.28) n=16, control mean 4.77 (±2.59) n=8, relative reduction in viral load, day 4.
|
|
viral load, 31.0% higher, relative load 1.31, p = 0.34, treatment mean 3.23 (±2.32) n=16, control mean 4.23 (±2.4) n=8, relative reduction in viral load, day 3.
|
|
viral load, 26.7% higher, relative load 1.27, p = 0.50, treatment mean 1.95 (±1.59) n=16, control mean 2.47 (±2.01) n=8, relative reduction in viral load, day 2.
|
|
viral load, 1.4% lower, relative load 0.99, p = 0.93, treatment mean 5.55 (±1.72) n=16, control mean 5.47 (±2.8) n=8, relative reduction in viral load, day 11.
|
|
risk of no viral clearance, 16.7% lower, RR 0.83, p = 1.00, treatment 5 of 16 (31.2%), control 3 of 8 (37.5%), NNT 16, day 5 and 11 viral clearance.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Khoo et al., 2 May 2025, Randomized Controlled Trial, United Kingdom, preprint, 31 authors, study period January 2023 - September 2023, average treatment delay 4.0 days, this trial uses multiple treatments in the treatment arm (combined with molnupiravir) - results of individual treatments may vary, trial NCT04746183 (history) (AGILE CST-8).
Contact: khoo@liverpool.ac.uk.
A randomised-controlled Phase I de-escalation trial of Molnupiravir and Nirmatrelvir/Ritonavir combination for mild-moderate SARS-CoV-2 infection
doi:10.1101/2025.05.01.25326797
Background The AGILE CST-8 (NCT04746183) Phase I de-escalation trial evaluated the safety and tolerability of combination molnupiravir and nirmatrelvir/ritonavir for mild-moderate COVID-19.
Methods Adult out-patients with SARS-CoV-2 infection within five days of symptoms were randomly assigned 2:1 to receive molnupiravir (starting at 800mg twice daily (BD) reducing to 600mg and 400mg if necessary) in combination with nirmatrelvir (300mg)/ritonavir (100mg) BD for 5 days versus Standard of care. Using a dose de-escalation, open-label, Bayesian adaptive Phase I trial a combination dose was considered unsafe if the probability of 30% or greater dose-limiting toxicity risk (DLT -the primary outcome) over standard of care was 25% or higher. Secondary endpoints included tolerability, clinical progression, pharmacokinetics and virological responses.
Findings Of 49 participants screened, 24 were enrolled (16 combination, 8 standard of care) between January 2023 and September 2023. For the primary endpoint, to day 11, no participant starting molnupiravir at 800mg (BD) in combination with nirmatrelvir/ritonavir reported a DLT by day 11 (primary endpoint) or by day 29; dose de-escalation was not required. No participants reported severe adverse events (grade>=3). Although proportion of swab PCR negativity at day 5 and day 11 were not statistically different, faster initial viral clearance was observed with treatment. Penetration of nirmatrelvir into saliva, nasal secretions and tears was 19%, 65% and 91% that of plasma.
Interpretation Molnupiravir in combination with nirmatrelvir/ritonavir was safe and well-tolerated; later phase trials should evaluate combination therapy at currently recommended doses for each drug.
Contributors SHK, GG, RF, TF, TJ, PM, LW contributed to study design. SHK, GG, GS, JN contributed to data analysis and interpretation. RF, SA, CJE led clinical conduct as principal investigators of the clinical sites. RL, RF, LW, CJE, SA, AB participated in clinical assessment and data collection. LJE, LD, VS, WG, CH, KB, AA contributed to study bioanalysis. EK, HER, MT, CM, JD, JG, JC contributed to study management and execution. DGL, AO, MJ contributed to the design of the AGILE platform. The manuscript was written by the authors, with SHK and GG as the overall lead authors. No one who is not an author contributed to writing the manuscript. All authors had full access to the data and GS and JN directly accessed and verified the underlying data reported in the manuscript. The authors assume responsibility for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. Blood and lymphatic system disorders Ear and labyrinth disorders 0 (0 General disorders and administration site conditions Infections and infestations 0 (0•0%) Musculoskeletal and connective tissue disorders Renal and urinary disorders 0 (0 Reproductive system and breast disorders Respiratory, thoracic and mediastinal disorders
References
Amara, Penchala, Else, Hale, Fitzgerald et al., The development and validation of a novel LC-MS/MS method for the simultaneous quantification of Molnupiravir and its metabolite ß-d-N4-hydroxycytidine in human plasma and saliva, J Pharm Biomed Anal
Anderson, Caubel, Rusnak, EPIC-HR Trial Investigators. Nirmatrelvir-Ritonavir and Viral Load Rebound in Covid-19, N Engl J Med
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Else, Watson, Tjia, Hughes, Siccardi et al., Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds, J Chromatogr B Analyt Technol Biomed Life Sci
Ewings, Saunders, Jaki, Mozgunov, Practical recommendations for implementing a Bayesian adaptive phase I design during a pandemic, BMC Med Res Methodol
Fiaschi, Biba, Varasi, Bartolini, Paletti et al., In Vitro Combinatorial Activity of Direct Acting Antivirals and Monoclonal Antibodies against the Ancestral B.1 and BQ.1.1 SARS-CoV-2 Viral Variants, Viruses
Fitzgerald, Dickinson, Else, Fletcher, Hale, Pharmacokinetics of ß-d-N4-Hydroxycytidine, the Parent Nucleoside of Prodrug Molnupiravir, in Nonplasma Compartments of Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection, Clin Infect Dis
Gandhi, Klein, Robertson, Peña-Hernández, Lin et al., De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report, Nat Commun
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Griffiths, Fitzgerald, Jaki, Corkhill, Marwood et al., AGILE-ACCORD: A Randomized, Multicentre, Seamless, Adaptive Phase I/II Platform Study to Determine the Optimal Dose, Safety and Efficacy of Multiple Candidate Agents for the Treatment of COVID-19: A structured summary of a study protocol for a randomised platform trial, Trials
Hogan, Duerr, Dimartino, Marier, Hochman et al., Remdesivir Resistance in Transplant Recipients With Persistent Coronavirus Disease 2019, Clin Infect Dis
Ip, Chu, Chan, Leung, Abdullah et al., Global prevalence of SARS-CoV-2 3CL protease mutations associated with nirmatrelvir or ensitrelvir resistance, EBioMedicine
Khoo, Fitzgerald, Fletcher, Ewings, Jaki et al., Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study, J Antimicrob Chemother
Khoo, Fitzgerald, Saunders, Middleton, Ahmad et al., Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial, Lancet Infect Dis
Lagevrio, Summary of Product Characteristics 19 th
Li, Choudhary, Boucau, Nathan, Speidel, SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency, Sci Transl Med
Marangoni, Antonello, Coppi, Palazzo, Nassi et al., Combination regimen of nirmatrelvir/ritonavir and molnupiravir for the treatment of persistent SARS-CoV-2 infection: A case report and a scoping review of the literature, Int J Infect Dis
Mikulska, Sepulcri, Dentone, Magne, Balletto et al., Triple Combination Therapy With 2 Antivirals and Monoclonal Antibodies for Persistent or Relapsed Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Patients, Clin Infect Dis
Nooruzzaman, Johnson, Finkelsztein, Caserta, Kodiyanplakkal, Emergence of transmissible SARS-CoV-2 variants with decreased sensitivity to antivirals in immunocompromised patients with persistent infections, Nat Commun
Paxlovid, Summary of Product Characteristics
Peluso, Swank, Goldberg, Lu, Dalhuisen et al., Plasma-based antigen persistence in the post-acute phase of COVID-19, Lancet Infect Dis
Penrice-Randal, Bentley, Sharma, Kirby, Donovan-Banfield et al., The effect of molnupiravir and nirmatrelvir on SARS-CoV-2 genome diversity in severe models of COVID-19, Microbiol Spectr
Rosenke, Lewis, Feldmann, Bohrnsen, Schwarz et al., Combined molnupiravir-nirmatrelvir treatment improves the inhibitory effect on SARS-CoV-2 in macaques, JCI Insight
Watson, Kissler, Day, Grad, White, Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies, Antimicrob Agents Chemother
Yamamoto, Taniguchi, Furukawa, Inaba, Niiyama et al., Nirmatrelvir Resistance in an Immunocompromised Patient with Persistent Coronavirus Disease, Viruses
Zhou, Long, Rosenke, Jarvis, Feldmann et al., Combined Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 Reduces Molnupiravir-Induced Mutagenicity and Prevents Selection for Nirmatrelvir/Ritonavir Resistance Mutations, J Infect Dis
Zuckerman, Bucris, Keidar-Friedman, Amsalem, Brosh-Nissimov, Nirmatrelvir Resistance-de Novo E166V/L50V Mutations in an Immunocompromised Patient Treated With Prolonged Nirmatrelvir/Ritonavir Monotherapy Leading to Clinical and Virological Treatment Failure-a Case Report, Clin Infect Dis
DOI record:
{
"DOI": "10.1101/2025.05.01.25326797",
"URL": "http://dx.doi.org/10.1101/2025.05.01.25326797",
"abstract": "<jats:p>Background The AGILE CST-8 (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04746183\">NCT04746183</jats:ext-link>) Phase I de-escalation trial evaluated the safety and tolerability of combination molnupiravir and nirmatrelvir/ritonavir for mild-moderate COVID-19. Methods Adult out-patients with SARS-CoV-2 infection within five days of symptoms were randomly assigned 2:1 to receive molnupiravir (starting at 800mg twice daily (BD) reducing to 600mg and 400mg if necessary) in combination with nirmatrelvir (300mg)/ritonavir (100mg) BD for 5 days versus Standard of care. Using a dose de-escalation, open-label, Bayesian adaptive Phase I trial a combination dose was considered unsafe if the probability of 30% or greater dose-limiting toxicity risk (DLT - the primary outcome) over standard of care was 25% or higher. Secondary endpoints included tolerability, clinical progression, pharmacokinetics and virological responses. Findings Of 49 participants screened, 24 were enrolled (16 combination, 8 standard of care) between January 2023 and September 2023. For the primary endpoint, to day 11, no participant starting molnupiravir at 800mg (BD) in combination with nirmatrelvir/ritonavir reported a DLT by day 11 (primary endpoint) or by day 29; dose de-escalation was not required. No participants reported severe adverse events (grade>=3). Although proportion of swab PCR negativity at day 5 and day 11 were not statistically different, faster initial viral clearance was observed with treatment. Penetration of nirmatrelvir into saliva, nasal secretions and tears was 19%, 65% and 91% that of plasma. Interpretation Molnupiravir in combination with nirmatrelvir/ritonavir was safe and well-tolerated; later phase trials should evaluate combination therapy at currently recommended doses for each drug. Funding UK National Institute for Health and Care Research, Medical Research Council (MR/V028391/1) and Wellcome Trust (221590/Z/20/Z).</jats:p>",
"accepted": {
"date-parts": [
[
2025,
5,
2
]
]
},
"author": [
{
"ORCID": "https://orcid.org/0000-0002-2769-0967",
"affiliation": [],
"authenticated-orcid": false,
"family": "Khoo",
"given": "Saye H",
"sequence": "first"
},
{
"affiliation": [],
"family": "FitzGerald",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Christopher J",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0400-8937",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ahmad",
"given": "Shazaad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Geoffrey",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0007-0078-3294",
"affiliation": [],
"authenticated-orcid": false,
"family": "Else",
"given": "Laura J",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0429-0186",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shaw",
"given": "Victoria",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6810-0284",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mozgunov",
"given": "Pavel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Northey",
"given": "Josh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dickinson",
"given": "Laura",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0004-3221-5820",
"affiliation": [],
"authenticated-orcid": false,
"family": "Knox",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buadi",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hale",
"given": "Colin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7443-4520",
"affiliation": [],
"authenticated-orcid": false,
"family": "Reynolds",
"given": "Helen E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Middleton",
"given": "Calley",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5758-0179",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bullock",
"given": "Katie",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1354-0826",
"affiliation": [],
"authenticated-orcid": false,
"family": "Walker",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tetlow",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lyon",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibney",
"given": "Jennifer",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1137-2948",
"affiliation": [],
"authenticated-orcid": false,
"family": "Amara",
"given": "Alieu",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1865-3195",
"affiliation": [],
"authenticated-orcid": false,
"family": "Greenhalf",
"given": "William",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0883-4160",
"affiliation": [],
"authenticated-orcid": false,
"family": "Burdon",
"given": "Abigail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dixon",
"given": "Jan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1096-188X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jaki",
"given": "Thomas",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8063-6399",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chiong",
"given": "Justin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7680-2200",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lalloo",
"given": "David G",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-9819-7651",
"affiliation": [],
"authenticated-orcid": false,
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobs",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fletcher",
"given": "Thomas",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-9579-8021",
"affiliation": [],
"authenticated-orcid": false,
"family": "Griffiths",
"given": "Gareth",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T22:20:17Z",
"timestamp": 1746224417000
},
"deposited": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T22:20:17Z",
"timestamp": 1746224417000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2025,
5,
3
]
],
"date-time": "2025-05-03T04:12:35Z",
"timestamp": 1746245555626,
"version": "3.40.4"
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
5,
2
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T00:00:00Z",
"timestamp": 1746144000000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2025.05.01.25326797",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
5,
2
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2025,
5,
2
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2025.05.01.25326797"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "A randomised-controlled Phase I de-escalation trial of Molnupiravir and Nirmatrelvir/Ritonavir combination for mild-moderate SARS-CoV-2 infection",
"type": "posted-content"
}
khoo3