Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study
et al., American Journal of Otolaryngology, doi:10.1016/j.amjoto.2020.102880, Jan 2021
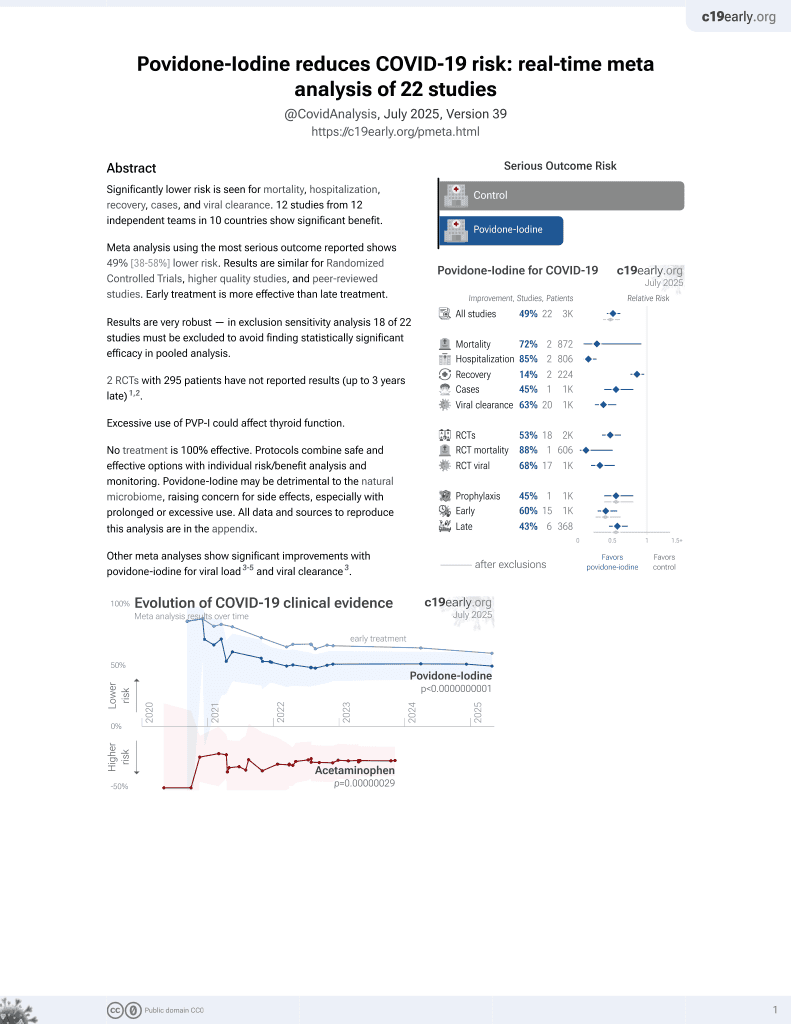
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
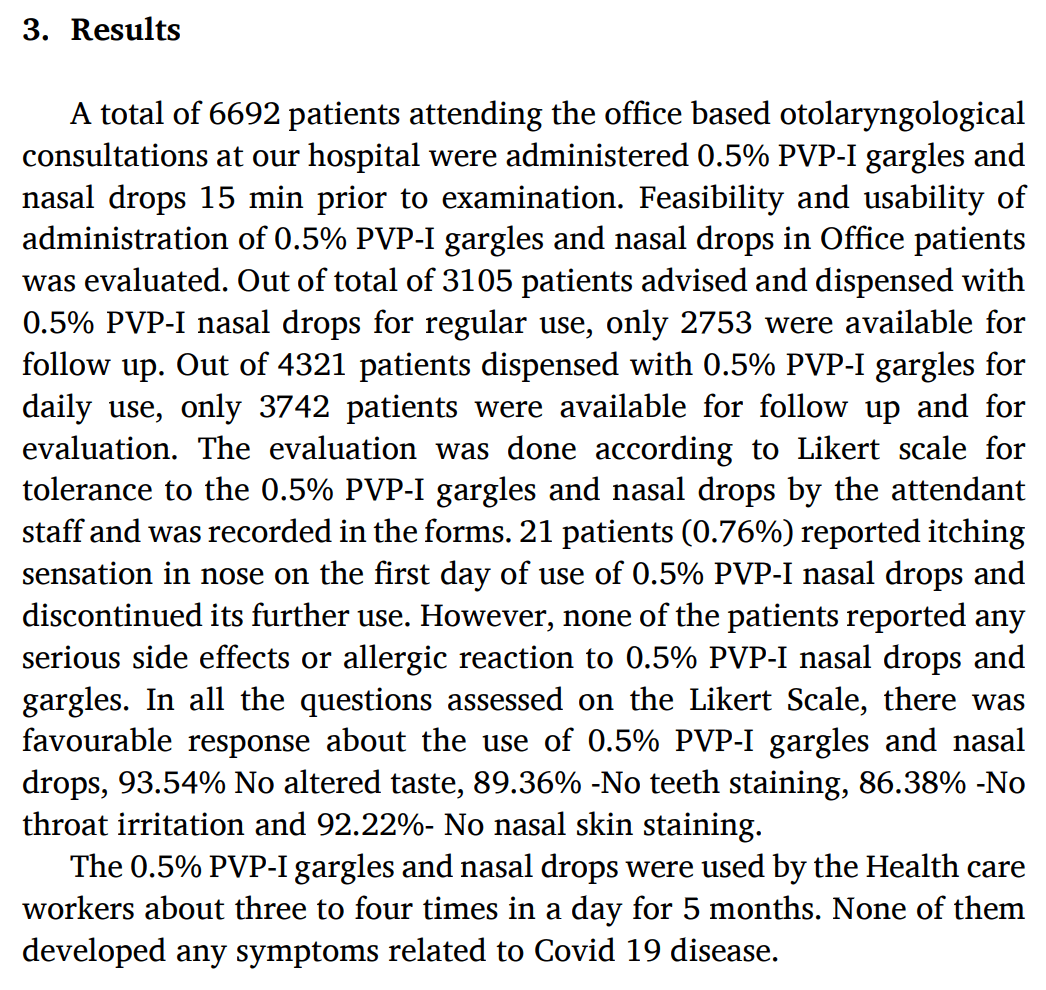
Study of the use of PVP-I gargles and nasal drops before and after ENT examinations with a total of 6,692 patients, finding high usability and good tolerance for use.
21 patients (0.76%) reported an itching sensation in the nose on the first day of use of 0.5% PVP-I nasal drops and discontinued its further use. However, none of the patients reported any serious side effects or allergic reaction to 0.5% PVP-I nasal drops and gargles.
The 0.5% PVP-I gargles and nasal drops were used by healthcare workers about three to four times in a day for 5 months. None of them developed any COVID-19 symptoms.
Khan et al., 3 Jan 2021, peer-reviewed, 2 authors.
Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study
American Journal of Otolaryngology, doi:10.1016/j.amjoto.2020.102880
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre -including this research content -immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
References
Ader, Paul, Reinhardt, Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function, J Clin Endocrinol Metab
Domingo, Farrales, Loya, Pura, Uy, The effect of 1% povidone iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene, J Philipp Dent Assoc
Eggers, Eickmann, Zorn, Rapid and effective Virucidal activity of povidoneiodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified Vaccinia virus Ankara (MVA), Infect Dis Ther, doi:10.1007/s40121-015-0091-9
Eggers, Infectious disease management and control with povidone iodine, Infect Dis Ther
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens, Infect Dis Ther, doi:10.1007/s40121-018-0200-7
Khan, Parab, 0.5% povidone iodine irrigation in otorhinolaryngology surgical practice during COVID 19 pandemic, Am J Otolaryngol, doi:10.1016/j.amjoto.2020.102687
Khan, Parab, Paranjape, Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in covid 19 pandemic, Am J Otolaryngol, doi:10.1016/j.amjoto.2020.102618
Lachapelle, Castel, Casado, Antiseptics in the era of bacterial resistance: a focus on povidone iodine, Future Med
Madan, Sequeira, Shenoy, Shetty, The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: a randomized control trial, J Cancer Res Ther
Mullings, Panchmatia, Samoy, Topical povidone-iodine as an adjunctive treatment for recalcitrant chronic rhinosinusitis, European Journal of Rhinology and Allergy, doi:10.5152/ejra.2019.166
Nagatake, Ahmed, Oishi, Prevention of respiratory infections by povidoneiodine gargle, Dermatology
Panchmatia, Payandeh, Al-Salman, The efficacy of diluted topical povidone-iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study, Eur Arch Otorhinolaryngol Published Online First, doi:10.1007/s00405-019-05628-w
Rackur, New aspects of mechanism of action of povidoneiodine, J Hosp Infect
Rahn, Adamietz, Boettcher, Schaefer, Reimer et al., Povidoneiodine to prevent mucositis in patients during antineoplastic radiochemotherapy, Dermatology
Reimer, Wichelhaus, Schafer, Antimicrobial effectiveness of povidoneiodine and consequences for new application areas, Dermatology
Rezapoor, Nicholson, Tabatabaee, Chen, Maltenfort et al., Povidone-iodine-based solutions for decolonization of nasal Staphylococcus aureus: a randomized, prospective, placebo-controlled study, J Arthroplasty, doi:10.1016/j.arth.2017.04.039
Sungnak, Huang, 'ecavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Wewalka, Stary, Bosse, Duerr, Reimer, Efficacy of povidone-iodine vaginal suppositories in the treatment of bacterial vaginosis, Dermatology
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1016/j.amjoto.2020.102880",
"ISSN": [
"0196-0709"
],
"URL": "http://dx.doi.org/10.1016/j.amjoto.2020.102880",
"alternative-id": [
"S0196070920305743"
],
"article-number": "102880",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "American Journal of Otolaryngology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.amjoto.2020.102880"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Inc. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Khan",
"given": "Mubarak Muhamed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Parab",
"given": "Sapna Ramkrishna",
"sequence": "additional"
}
],
"container-title": "American Journal of Otolaryngology",
"container-title-short": "American Journal of Otolaryngology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
1,
8
]
],
"date-time": "2021-01-08T03:46:44Z",
"timestamp": 1610077604000
},
"deposited": {
"date-parts": [
[
2023,
1,
13
]
],
"date-time": "2023-01-13T20:56:57Z",
"timestamp": 1673643417000
},
"indexed": {
"date-parts": [
[
2024,
5,
4
]
],
"date-time": "2024-05-04T15:13:51Z",
"timestamp": 1714835631745
},
"is-referenced-by-count": 10,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
3
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
1
]
],
"date-time": "2021-03-01T00:00:00Z",
"timestamp": 1614556800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
1
]
],
"date-time": "2021-03-01T00:00:00Z",
"timestamp": 1614556800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
1
]
],
"date-time": "2021-03-01T00:00:00Z",
"timestamp": 1614556800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
1
]
],
"date-time": "2021-03-01T00:00:00Z",
"timestamp": 1614556800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
1
]
],
"date-time": "2021-03-01T00:00:00Z",
"timestamp": 1614556800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
1
]
],
"date-time": "2021-03-01T00:00:00Z",
"timestamp": 1614556800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0196070920305743?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0196070920305743?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102880",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
3
]
]
},
"published-print": {
"date-parts": [
[
2021,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1007/s40121-018-0200-7",
"article-title": "In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens",
"author": "Eggers",
"doi-asserted-by": "crossref",
"first-page": "249",
"issue": "2",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.amjoto.2020.102880_bb0005",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"article-title": "Rapid and effective Virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified Vaccinia virus Ankara (MVA)",
"author": "Eggers",
"doi-asserted-by": "crossref",
"first-page": "491",
"issue": "4",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.amjoto.2020.102880_bb0010",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1016/j.amjoto.2020.102618",
"article-title": "Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in covid 19 pandemic",
"author": "Khan",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Am J Otolaryngol",
"key": "10.1016/j.amjoto.2020.102880_bb0015",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1016/j.amjoto.2020.102687",
"article-title": "0.5% povidone iodine irrigation in otorhinolaryngology surgical practice during COVID 19 pandemic",
"author": "Khan",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Am J Otolaryngol",
"key": "10.1016/j.amjoto.2020.102880_bb0020",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "1177",
"issue": "12",
"journal-title": "N Engl J Med",
"key": "10.1016/j.amjoto.2020.102880_bb0025",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes",
"author": "Sungnak",
"doi-asserted-by": "crossref",
"first-page": "681",
"issue": "5",
"journal-title": "Nat Med",
"key": "10.1016/j.amjoto.2020.102880_bb0030",
"volume": "26",
"year": "2020"
},
{
"article-title": "Antiseptics in the era of bacterial resistance: a focus on povidone iodine",
"author": "Lachapelle",
"first-page": "579",
"journal-title": "Future Med",
"key": "10.1016/j.amjoto.2020.102880_bb0035",
"volume": "10",
"year": "2013"
},
{
"article-title": "The effect of 1% povidone iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene",
"author": "Domingo",
"first-page": "31",
"journal-title": "J Philipp Dent Assoc",
"key": "10.1016/j.amjoto.2020.102880_bb0040",
"volume": "48",
"year": "1996"
},
{
"DOI": "10.1159/000057731",
"article-title": "Efficacy of povidone-iodine vaginal suppositories in the treatment of bacterial vaginosis",
"author": "Wewalka",
"doi-asserted-by": "crossref",
"first-page": "79",
"issue": "Suppl. 1",
"journal-title": "Dermatology",
"key": "10.1016/j.amjoto.2020.102880_bb0045",
"volume": "204",
"year": "2002"
},
{
"article-title": "Infectious disease management and control with povidone iodine",
"author": "Eggers",
"first-page": "1",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.amjoto.2020.102880_bb0050",
"volume": "1",
"year": "2019"
},
{
"DOI": "10.1016/S0195-6701(85)80041-4",
"article-title": "New aspects of mechanism of action of povidoneiodine",
"author": "Rackur",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "J Hosp Infect",
"key": "10.1016/j.amjoto.2020.102880_bb0055",
"volume": "6",
"year": "1985"
},
{
"DOI": "10.1159/000057722",
"article-title": "Prevention of respiratory infections by povidone-iodine gargle",
"author": "Nagatake",
"doi-asserted-by": "crossref",
"first-page": "32",
"issue": "1",
"journal-title": "Dermatology",
"key": "10.1016/j.amjoto.2020.102880_bb0060",
"volume": "204",
"year": "2002"
},
{
"DOI": "10.4103/0973-1482.39597",
"article-title": "The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: a randomized control trial",
"author": "Madan",
"doi-asserted-by": "crossref",
"first-page": "3",
"issue": "1",
"journal-title": "J Cancer Res Ther",
"key": "10.1016/j.amjoto.2020.102880_bb0065",
"volume": "4",
"year": "2008"
},
{
"DOI": "10.1159/000246032",
"article-title": "Povidone-iodine to prevent mucositis in patients during antineoplastic radiochemotherapy",
"author": "Rahn",
"doi-asserted-by": "crossref",
"first-page": "57",
"issue": "2",
"journal-title": "Dermatology",
"key": "10.1016/j.amjoto.2020.102880_bb0070",
"volume": "195",
"year": "1997"
},
{
"DOI": "10.1210/jcem-66-3-632",
"article-title": "Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function",
"author": "Ader",
"doi-asserted-by": "crossref",
"first-page": "632",
"issue": "3",
"journal-title": "J Clin Endocrinol Metab",
"key": "10.1016/j.amjoto.2020.102880_bb0075",
"volume": "66",
"year": "1988"
},
{
"DOI": "10.1016/j.arth.2017.04.039",
"article-title": "Povidone-iodine-based solutions for decolonization of nasal Staphylococcus aureus: a randomized, prospective, placebo-controlled study",
"author": "Rezapoor",
"doi-asserted-by": "crossref",
"first-page": "2815",
"issue": "9",
"journal-title": "J Arthroplasty",
"key": "10.1016/j.amjoto.2020.102880_bb0080",
"volume": "32",
"year": "2017"
},
{
"DOI": "10.1007/s00405-019-05628-w",
"doi-asserted-by": "crossref",
"key": "10.1016/j.amjoto.2020.102880_bb0085",
"unstructured": "Panchmatia R, Payandeh J, Al-Salman R, et al. The efficacy of diluted topical povidone-iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study. Eur Arch Otorhinolaryngol Published Online First: 2019. doi:https://doi.org/10.1007/s00405-019-05628-w."
},
{
"DOI": "10.5152/ejra.2019.166",
"article-title": "Topical povidone-iodine as an adjunctive treatment for recalcitrant chronic rhinosinusitis",
"author": "Mullings",
"doi-asserted-by": "crossref",
"journal-title": "European Journal of Rhinology and Allergy",
"key": "10.1016/j.amjoto.2020.102880_bb0090",
"year": "2019"
},
{
"DOI": "10.1159/000057738",
"article-title": "Antimicrobial effectiveness of povidone-iodine and consequences for new application areas",
"author": "Reimer",
"doi-asserted-by": "crossref",
"first-page": "114",
"issue": "1",
"journal-title": "Dermatology",
"key": "10.1016/j.amjoto.2020.102880_bb0095",
"volume": "204",
"year": "2002"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0196070920305743"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "42"
}