Effect of Spironolactone on COVID-19 in Patients With Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea
et al., Frontiers in Medicine, doi:10.3389/fmed.2021.629176, Feb 2021
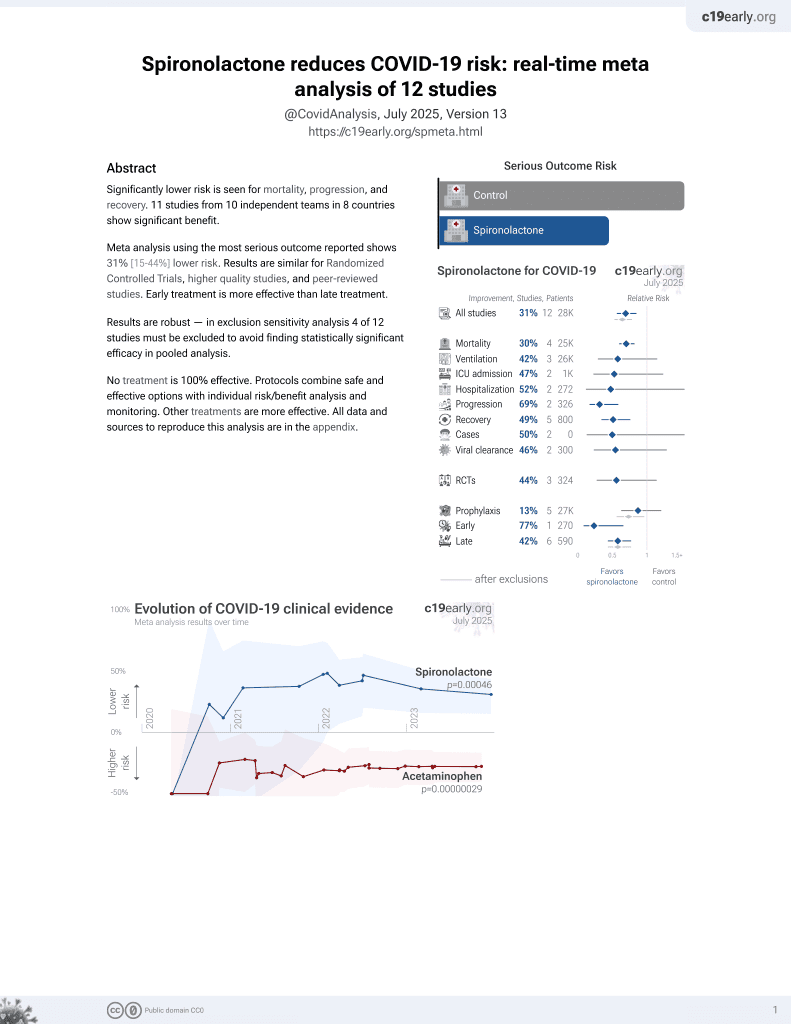
37th treatment shown to reduce risk in
February 2022, now with p = 0.00046 from 12 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
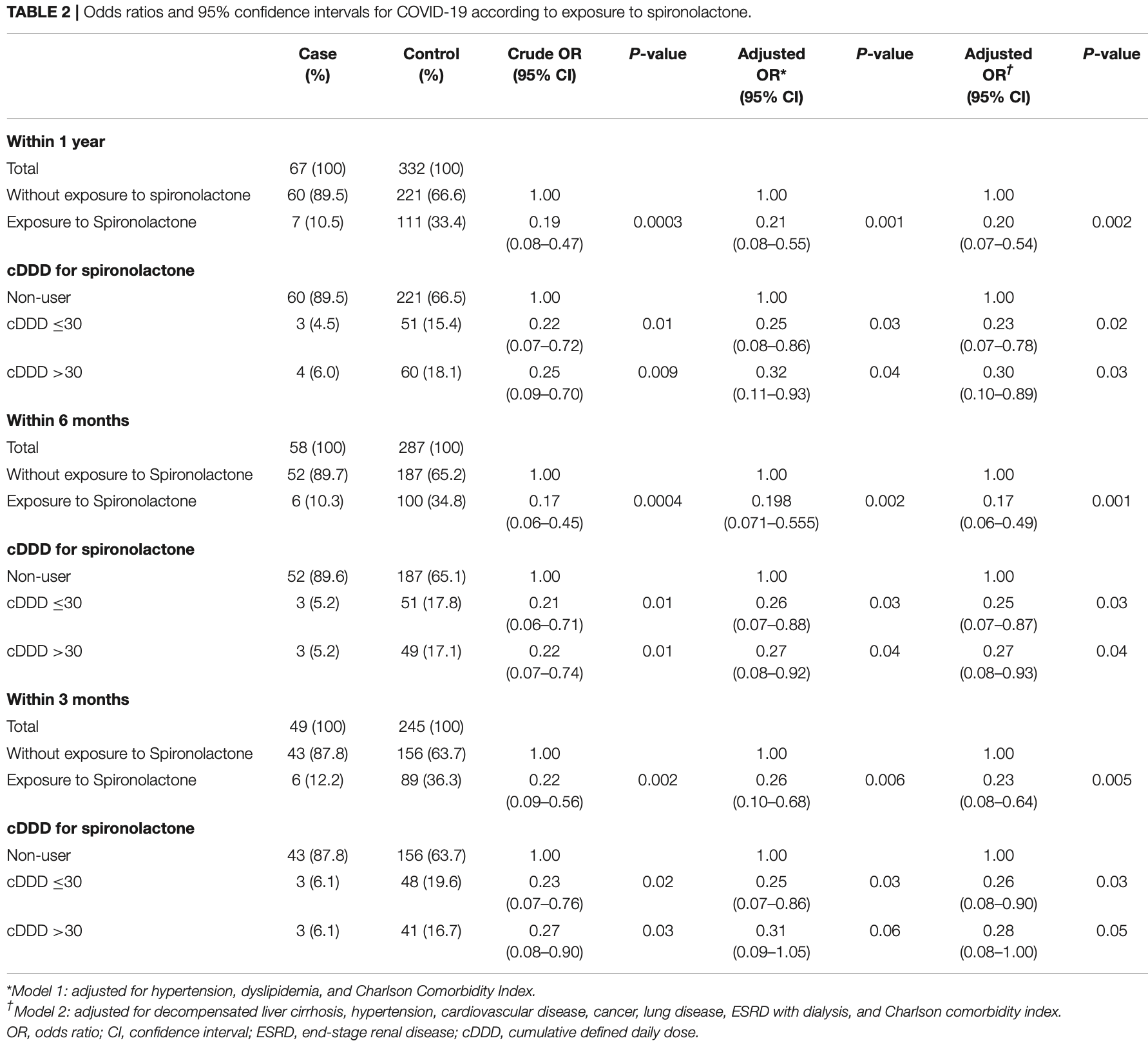
Retrospective 6,462 liver cirrhosis patients in South Korea, with 67 COVID+ cases, showing significantly lower cases with spironolactone treatment. Death and ICU results per group are not provided.
|
risk of case, 77.0% lower, OR 0.23, p = 0.005, treatment 6 of 49 (12.2%) cases,
89 of 245 (36.3%) controls, NNT 6.5, case control OR, model 2, within 3 months.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jeon et al., 23 Feb 2021, retrospective, South Korea, peer-reviewed, 3 authors.
Effect of Spironolactone on COVID-19 in Patients With Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea
Frontiers in Medicine, doi:10.3389/fmed.2021.629176
Purpose: On the basis that spironolactone is involved in ACE2 expression and TMPRSS2 activity, previous studies have suggested that spironolactone may influence the infectivity of COVID-19. Research has suggested that cell entry of SARS-CoV-2, the virus that induces COVID-19, is associated with the ACE2 receptor and TMPRSS2. The purpose of this study was to investigate whether spironolactone has a protective effect against COVID-19 and the development of associated complications in patients with liver cirrhosis. Methods: We conducted a nationwide case-control study on liver cirrhosis patients with or without COVID-19 from the population-based data acquired from the National Health Insurance Systems of Republic of Korea. After 1:5 case-control matching, multivariable adjusted conditional logistic regression analysis was performed. Results: Among the patients with liver cirrhosis, the case group with COVID-19 was found to be significantly less exposed to spironolactone compared with the control group without COVID-19. The adjusted odds ratio (OR) and 95% confidence interval (CI) between the two groups was 0.20 (0.07-0.54). In addition, regardless of cumulative dose of spironolactone, exposure to spironolactone was associated with lower COVID-19 infection. In terms of the development of complications due to COVID-19, spironolactone did not show any significant association between the patients with and without complications (P = 0.43). The adjusted OR and 95% CI between the two groups was 1.714 (0.246-11.938).
Conclusion: We conclude that spironolactone may reduce susceptibility to COVID-19 but does not affect the development of its associated complications; however, further studies are needed to confirm the exact association between spironolactone and COVID-19 infection.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by Institutional Review Board of Asan Medical Center, Seoul, Republic of Korea (IRB number: 2020-1153). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AUTHOR CONTRIBUTIONS DJ, MS, and JC were responsible for the conception and design of the study, acquisition, analysis and interpretation of the data, and drafting of the manuscript. MS performed the statistical analyses. All authors have full access to all data used in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and approved the final version of the manuscript.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed. 2021.629176/full#supplementary-material
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alhazzani, Møller, Arabi, Loeb, Gong, Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive Care Med, doi:10.1007/s00134-020-06022-5
Bajaj, Garcia-Tsao, Biggins, Kamath, Wong et al., Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort, Gut, doi:10.1136/gutjnl-2020-322118
Broulik, Stárka, Antiandrogenic and antirenotropic effect of spironolactone, Endokrinologie
Cadegiani, Goren, Wambier, Spironolactone may provide protection from SARS-CoV-2: targeting androgens, angiotensin converting enzyme 2 (ACE2), and renin-angiotensin-aldosterone system (RAAS), Med Hypotheses, doi:10.1016/j.mehy.2020.110112
Cadegiani, Wambier, Goren, Spironolactone: An Antiandrogenic and Anti-hypertensive Drug That May Provide Protection Against the Novel Coronavirus (SARS-CoV-2) Induced Acute Respiratory Distress Syndrome (ARDS) in COVID-19, Front Med, doi:10.3389/fmed.2020.00453
Cdc Covid-, 19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)-United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6912e2
Chung, Chung, Kim, Liver cirrhosis and cancer: comparison of mortality, Hepatol Int, doi:10.1007/s12072-018-9850-5
De Groot, Beckerman, Lankhorst, Bouter, How to measure comorbidity. a critical review of available methods, J Clin Epidemiol, doi:10.1016/S0895-4356(02)00585-1
Epstein, Da, Aldosterone blockers (mineralocorticoid receptor antagonism) and potassium-sparing diuretics, J Clin Hypertens, doi:10.1111/j.1751-7176.2011.00511.x
Hoffmann, Kleine-Weber, Krüger, Müller, Drosten et al., The novel coronavirus 2019 (2019-nCoV) uses the SARScoronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells, bioRxiv, doi:10.1101/2020.01.31.929042
Jung, Choi, You, Kim, Association of renin-angiotensinaldosterone system inhibitors with coronavirus disease 2019 (COVID-19)-related outcomes in Korea: a nationwide population-based cohort study, Clin Infect Dis, doi:10.1093/cid/ciaa624
Keidar, Gamliel-Lazarovich, Kaplan, Pavlotzky, Hamoud et al., Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients, Circ Res, doi:10.1161/01.RES.0000187500.24964.7A
Kim, Jeong, Byun, Cho, Park et al., Evaluation of COVID-19 epidemic outbreak caused by temporal contact-increase in South Korea, Int J Infect Dis, doi:10.1016/j.ijid.2020.05.036
Kuba, Imai, Rao, Gao, Guo et al., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirusinduced lung injury, Nat Med, doi:10.1038/nm1267
Li, Zhou, Yang, You, Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor, Pharmacol Res, doi:10.1016/j.phrs.2020.104833
Liaudet, Szabo, Blocking mineralocorticoid receptor with spironolactone may have a wide range of therapeutic actions in severe COVID-19 disease, Critical Care, doi:10.1186/s13054-020-03055-6
Rajgor, Lee, Archuleta, Bagdasarian, Quek, The many estimates of the COVID-19 case fatality rate, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30244-9
Sanyaolu, Okorie, Marinkovic, Patidar, Younis et al., Comorbidity and its impact on patients with COVID-19, SN Compr Clin Med, doi:10.1007/s42399-020-00363-4
Shim, Tariq, Choi, Lee, Chowell, Transmission potential and severity of COVID-19 in South Korea, Int J Infect Dis, doi:10.1016/j.ijid.2020.03.031
Yang, Kor, Hsieh, Long-term effects of spironolactone on kidney function and hyperkalemia-associated hospitalization in patients with chronic kidney disease, J Clin Med, doi:10.3390/jcm7110459
DOI record:
{
"DOI": "10.3389/fmed.2021.629176",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2021.629176",
"abstract": "<jats:p><jats:bold>Purpose:</jats:bold> On the basis that spironolactone is involved in ACE2 expression and TMPRSS2 activity, previous studies have suggested that spironolactone may influence the infectivity of COVID-19. Research has suggested that cell entry of SARS-CoV-2, the virus that induces COVID-19, is associated with the ACE2 receptor and TMPRSS2. The purpose of this study was to investigate whether spironolactone has a protective effect against COVID-19 and the development of associated complications in patients with liver cirrhosis.</jats:p><jats:p><jats:bold>Methods:</jats:bold> We conducted a nationwide case-control study on liver cirrhosis patients with or without COVID-19 from the population-based data acquired from the National Health Insurance Systems of Republic of Korea. After 1:5 case-control matching, multivariable adjusted conditional logistic regression analysis was performed.</jats:p><jats:p><jats:bold>Results:</jats:bold> Among the patients with liver cirrhosis, the case group with COVID-19 was found to be significantly less exposed to spironolactone compared with the control group without COVID-19. The adjusted odds ratio (OR) and 95% confidence interval (CI) between the two groups was 0.20 (0.07–0.54). In addition, regardless of cumulative dose of spironolactone, exposure to spironolactone was associated with lower COVID-19 infection. In terms of the development of complications due to COVID-19, spironolactone did not show any significant association between the patients with and without complications (<jats:italic>P</jats:italic> = 0.43). The adjusted OR and 95% CI between the two groups was 1.714 (0.246–11.938).</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> We conclude that spironolactone may reduce susceptibility to COVID-19 but does not affect the development of its associated complications; however, further studies are needed to confirm the exact association between spironolactone and COVID-19 infection.</jats:p>",
"alternative-id": [
"10.3389/fmed.2021.629176"
],
"author": [
{
"affiliation": [],
"family": "Jeon",
"given": "Dongsub",
"sequence": "first"
},
{
"affiliation": [],
"family": "Son",
"given": "Minkook",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choi",
"given": "Jonggi",
"sequence": "additional"
}
],
"container-title": [
"Frontiers in Medicine"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
2,
23
]
],
"date-time": "2021-02-23T05:34:06Z",
"timestamp": 1614058446000
},
"deposited": {
"date-parts": [
[
2021,
2,
23
]
],
"date-time": "2021-02-23T05:34:12Z",
"timestamp": 1614058452000
},
"indexed": {
"date-parts": [
[
2022,
3,
31
]
],
"date-time": "2022-03-31T02:28:37Z",
"timestamp": 1648693717613
},
"is-referenced-by-count": 5,
"issn-type": [
{
"type": "electronic",
"value": "2296-858X"
}
],
"issued": {
"date-parts": [
[
2021,
2,
23
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
23
]
],
"date-time": "2021-02-23T00:00:00Z",
"timestamp": 1614038400000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2021.629176/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
2,
23
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
23
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30244-9",
"article-title": "The many estimates of the COVID-19 case fatality rate",
"author": "Rajgor",
"doi-asserted-by": "publisher",
"first-page": "776",
"journal-title": "Lancet Infect Dis",
"key": "B1",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6912e2",
"article-title": "Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020",
"author": "CDC",
"doi-asserted-by": "publisher",
"first-page": "343",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "B2",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1111/j.1751-7176.2011.00511.x",
"article-title": "Aldosterone blockers (mineralocorticoid receptor antagonism) and potassium-sparing diuretics",
"author": "Epstein",
"doi-asserted-by": "publisher",
"first-page": "644",
"journal-title": "J Clin Hypertens",
"key": "B3",
"volume": "13",
"year": "2011"
},
{
"DOI": "10.1016/j.mehy.2020.110112",
"article-title": "Spironolactone may provide protection from SARS-CoV-2: targeting androgens, angiotensin converting enzyme 2 (ACE2), and renin-angiotensin-aldosterone system (RAAS)",
"author": "Cadegiani",
"doi-asserted-by": "publisher",
"first-page": "110112",
"journal-title": "Med Hypotheses",
"key": "B4",
"volume": "143",
"year": "2020"
},
{
"article-title": "Antiandrogenic and antirenotropic effect of spironolactone",
"author": "Broulik",
"first-page": "35",
"journal-title": "Endokrinologie",
"key": "B5",
"volume": "68",
"year": "1976"
},
{
"DOI": "10.1161/01.RES.0000187500.24964.7A",
"article-title": "Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients",
"author": "Keidar",
"doi-asserted-by": "publisher",
"first-page": "946",
"journal-title": "Circ Res",
"key": "B6",
"volume": "97",
"year": "2005"
},
{
"DOI": "10.1038/nm1267",
"article-title": "A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury",
"author": "Kuba",
"doi-asserted-by": "publisher",
"first-page": "875",
"journal-title": "Nat Med",
"key": "B7",
"volume": "11",
"year": "2005"
},
{
"DOI": "10.1101/2020.01.31.929042",
"article-title": "The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "bioRxiv",
"key": "B8",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.104833",
"article-title": "Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "104833",
"journal-title": "Pharmacol Res",
"key": "B9",
"volume": "157",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2020.00453",
"article-title": "Spironolactone: An Anti-androgenic and Anti-hypertensive Drug That May Provide Protection Against the Novel Coronavirus (SARS-CoV-2) Induced Acute Respiratory Distress Syndrome (ARDS) in COVID-19",
"author": "Cadegiani",
"doi-asserted-by": "publisher",
"first-page": "453",
"journal-title": "Front Med",
"key": "B10",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03055-6",
"article-title": "Blocking mineralocorticoid receptor with spironolactone may have a wide range of therapeutic actions in severe COVID-19 disease",
"author": "Liaudet",
"doi-asserted-by": "publisher",
"first-page": "318",
"journal-title": "Critical Care",
"key": "B11",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1007/s12072-018-9850-5",
"article-title": "Liver cirrhosis and cancer: comparison of mortality",
"author": "Chung",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Hepatol Int",
"key": "B12",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1016/j.ijid.2020.05.036",
"article-title": "Evaluation of COVID-19 epidemic outbreak caused by temporal contact-increase in South Korea",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "454",
"journal-title": "Int J Infect Dis",
"key": "B13",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.03.031",
"article-title": "Transmission potential and severity of COVID-19 in South Korea",
"author": "Shim",
"doi-asserted-by": "publisher",
"first-page": "339",
"journal-title": "Int J Infect Dis",
"key": "B14",
"volume": "93",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06022-5",
"article-title": "Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19)",
"author": "Alhazzani",
"doi-asserted-by": "publisher",
"first-page": "854",
"journal-title": "Intensive Care Med",
"key": "B15",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa624",
"article-title": "Association of renin-angiotensin-aldosterone system inhibitors with coronavirus disease 2019 (COVID-19)-related outcomes in Korea: a nationwide population-based cohort study",
"author": "Jung",
"doi-asserted-by": "publisher",
"first-page": "2121",
"journal-title": "Clin Infect Dis",
"key": "B16",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.3390/jcm7110459",
"article-title": "Long-term effects of spironolactone on kidney function and hyperkalemia-associated hospitalization in patients with chronic kidney disease",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "459",
"journal-title": "J Clin Med",
"key": "B17",
"volume": "7",
"year": "2018"
},
{
"key": "B18",
"unstructured": ""
},
{
"key": "B19",
"unstructured": "People of Any Age With Underlying Medical Conditions"
},
{
"DOI": "10.1016/S0895-4356(02)00585-1",
"article-title": "How to measure comorbidity. a critical review of available methods",
"author": "de Groot",
"doi-asserted-by": "publisher",
"first-page": "221",
"journal-title": "J Clin Epidemiol",
"key": "B20",
"volume": "56",
"year": "2003"
},
{
"DOI": "10.1136/gutjnl-2020-322118",
"article-title": "Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort",
"author": "Bajaj",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Gut",
"key": "B21",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.1007/s42399-020-00363-4",
"article-title": "Comorbidity and its impact on patients with COVID-19",
"author": "Sanyaolu",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "SN Compr Clin Med",
"key": "B22",
"volume": "25",
"year": "2020"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2021.629176/full"
}
},
"score": 1,
"short-container-title": [
"Front. Med."
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Effect of Spironolactone on COVID-19 in Patients With Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "8"
}
jeon