Safety and Effectiveness of High-Dose Vitamin C in Patients with COVID-19; A Randomized Controlled open-label Clinical Trial
et al., Research Square, doi:10.21203/rs.3.rs-139942/v1, Jan 2021
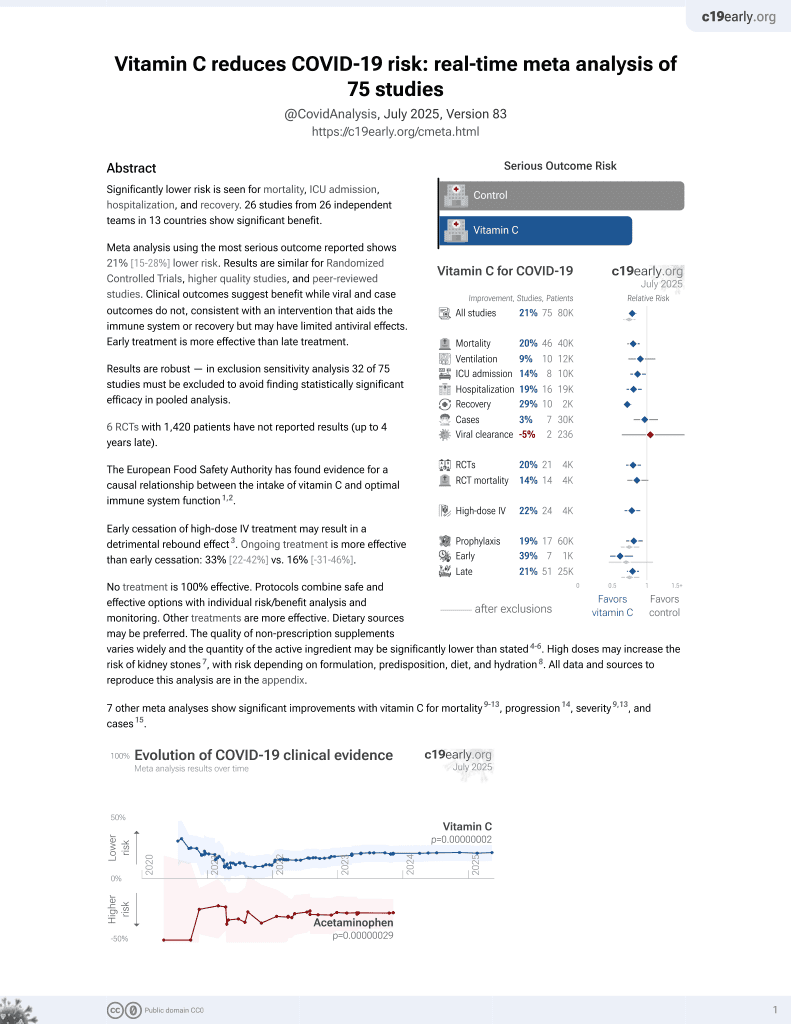
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small late stage RCT for the addition of vitamin C to HCQ and lopinavir/ritonavir, with 30 treatment and 30 control patients, finding a significant reduction in temperature and a significant improvement in oxygenation after 3 days in the vitamin C group. However, hospitalization time was longer and there was no significant difference in mortality.
This is the 3rd of 20 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0016.
This is the 10th of 73 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000076.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 3 of 30 (10.0%), control 3 of 30 (10.0%).
|
|
risk of mechanical ventilation, 25.0% higher, RR 1.25, p = 1.00, treatment 5 of 30 (16.7%), control 4 of 30 (13.3%).
|
|
hospitalization time, 30.8% higher, relative time 1.31, p = 0.03, treatment 30, control 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
JamaliMoghadamSiahkali et al., 9 Jan 2021, Randomized Controlled Trial, Iran, preprint, 17 authors, study period April 2020 - May 2020, dosage 1500mg qid days 1-5.
Background: To assess the effectiveness of vitamin C treatment against coronavirus disease 2019 (COVID-19) Methods: An open-label, randomized, and controlled trial was conducted on patients with severe COVID-19 infection. The case and control treatment groups each consisted of 30 patients. The control group received lopinavir/ritonavir and hydroxychloroquine and the case group received high-dose of vitamin C (six gr daily) added to the same regimen. Results: There were no statistically signi cant differences between two groups with respect to age and gender, laboratory results, and underlying diseases. On the 3 rd day of hospitalization, the mean core body temperatures was signi cantly lower and SpO2 was higher In the case group (p value = 0.001, and 0.014, respectively). The median length of hospitalization in case group which was signi cantly longer than the control group (8.5 days vs. 6.5 days) (p value = 0.0280). There was no signi cant difference in SpO2 levels at discharge time, the length of ICU stay, and mortality between the two groups. Conclusions: We did not nd signi cantly better outcomes in the group who were treated with high-dose vitamin C in addition to the main treatment regimen at discharge. Trial registration: The project was registered by Iranian Registry of Clinical Trials.
References
Asadollahi-Amin, Hasibi, Ghadimi, Lung involvement found on chest ct scan in a pre-symptomatic person with SARS-CoV-2 infection: a case report, Tropical
Berger, Oudemans-Van Straaten, Vitamin C supplementation in the critically ill patient, Current Opinion in Clinical Nutrition Metabolic Care
Borrelli, Roux-Lombard, Grau, Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk, Critical care medicine
Carr, Vitamin C administration in the critically ill: a summary of recent meta-analyses, Crit Care
Cheng, Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)?, Medicine in Drug Discovery
Du, Yuan, Sun, Therapeutic e cacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms, World Journal of gastroenterology
Fisher, Kraskauskas, Martin, Attenuation of sepsis-induced organ injury in mice by vitamin C, Journal of Parenteral Enteral Nutrition
Fisher, Seropian, Kraskauskas, Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury, Critical care medicine
Fowler, Kim, Lepler, Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome, World Journal of Critical Care Medicine
Furuya, Uozaki, Yamasaki, Antiviral effects of ascorbic and dehydroascorbic acids in vitro, Int J Mol Med
Hemilä, Chalker, Vitamin C as a possible therapy for COVID-19, Infection & Chemotherapy
Hemilä, Chalker, Vitamin C can shorten the length of stay in the ICU: a meta-analysis, Nutrients
Hemilä, Chalker, Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis, Journal of Intensive Care
Hemilä, Vitamin C and SARS coronavirus, J Antimicrob Chemother
Hickey, Saul, Vitamin C: The Real Story: the Remarkable and Controversial Healing Factor
Integrative, PDQ Cancer Information Summaries
Kennes, Dumont, Brohee, Effects of ascorbate on leucocytes-Part IV. Increased neutrophil function and clinical improvement after oral ascorbate in 2 patients with chronic granulomatous disease, South African Medical Journal
Li, Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis, Crit Care
Messina, Polito, Monda, Functional role of dietary intervention to improve the outcome of COVID-19: A hypothesis of work, Int J Mol Sci
Mikirova, Casciari, Riordan, Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of in ammation and disease in cancer patients, Journal of translational medicine
Mikirova, Hunninghake, Effect of high dose vitamin C on Epstein-Barr viral infection, journal of experimental clinical research
Mousavi, Bereswill, Heimesaat, Immunomodulatory and antimicrobial effects of vitamin C, European Journal of Microbiology Immunology
Padayatty, Sun, Wang, Vitamin C pharmacokinetics: implications for oral and intravenous use, Ann Intern Med
Panel, Shanghai, Expert Coronary Disease Comprehensive Treatment Consensus
Polidori, Mecocci, Levine, Short-term and long-term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation, Arch Biochem Biophys
Putzu, Daems, Lopez-Delgado, The effect of vitamin C on clinical outcome in critically ill patients: a systematic review with meta-analysis of randomized controlled trials, Critical care medicine
Rice, Russo-Menna, Differential compartmentalization of brain ascorbate and glutathione between neurons and glia, Neuroscience
Riordan, Jackson, Riordan, High-dose intravenous vitamin C in the treatment of a patient with renal cell carcinoma of the kidney, Journal of orthomolecular medicine
Sherman, Keaney, Jr, Biegelsen, Pharmacological of ascorbic acid are required for the bene cial effect on endothelial vasomotor function in hypertension, Hypertension
Syed, Knowlson, Sculthorpe, Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis, Journal of translational medicine
Tan, Hong, Saha, Medications in COVID-19 patients: summarizing the current literature from an orthopaedic perspective, International Orthopaedics
Truwit, Hite, Morris, Effect of vitamin C infusion on organ failure and biomarkers of in ammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial, Jama
Wu, Wang, Kuo, An update on current therapeutic drugs treating COVID-19, Current Pharmacology Reports
Zhang, Jativa, Vitamin C supplementation in the critically ill: A systematic review and meta-analysis, SAGE open medicine
DOI record:
{
"DOI": "10.21203/rs.3.rs-139942/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-139942/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p><jats:bold>Background: </jats:bold>To assess the effectiveness of vitamin C treatment against coronavirus disease 2019 (COVID-19)<jats:bold>Methods</jats:bold>: An open-label, randomized, and controlled trial was conducted on patients with severe COVID-19 infection. The case and control treatment groups each consisted of 30 patients. The control group received lopinavir/ritonavir and hydroxychloroquine and the case group received high-dose of vitamin C (six gr daily) added to the same regimen. <jats:bold>Results</jats:bold>: There were no statistically significant differences between two groups with respect to age and gender, laboratory results, and underlying diseases. On the 3<jats:sup>rd</jats:sup> day of hospitalization, the mean core body temperatures was significantly lower and SpO2 was higher In the case group (p value = 0.001, and 0.014, respectively). The median length of hospitalization in case group which was significantly longer than the control group (8.5 days <jats:italic>vs.</jats:italic> 6.5 days) (p value = 0.0280). There was no significant difference in SpO2 levels at discharge time, the length of ICU stay, and mortality between the two groups.<jats:bold>Conclusions: </jats:bold>We did not find significantly better outcomes in the group who were treated with high-dose vitamin C in addition to the main treatment regimen at discharge.<jats:bold>Trial registration:</jats:bold> The project was registered by Iranian Registry of Clinical Trials.IRCT20200411047025N1</jats:p>",
"accepted": {
"date-parts": [
[
2021,
1,
3
]
]
},
"author": [
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "JamaliMoghadamSiahkali",
"given": "Saeidreza",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Zarezade",
"given": "Besharat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Koolaji",
"given": "Sogol",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Alinaghi",
"given": "Seyed Ahmad Seyed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Zendehdel",
"given": "Abolfazl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Tabarestani",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Moghadam",
"given": "Ehsan Sekhavati",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Abbasian",
"given": "Ladan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Manshavi",
"given": "Sayed Ali Dehghan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Salehi",
"given": "Mohamadreza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Hasannezhad",
"given": "Malihe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Ghaderkhani",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Meidani",
"given": "Mohsen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Salahshour",
"given": "Faeze",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Jafari",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Institute"
}
],
"family": "Manafi",
"given": "Navid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tehran University of Medical Sciences"
}
],
"family": "Ghiasvand",
"given": "Fereshteh",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
1,
9
]
],
"date-time": "2021-01-09T18:38:17Z",
"timestamp": 1610217497000
},
"deposited": {
"date-parts": [
[
2022,
7,
29
]
],
"date-time": "2022-07-29T00:52:16Z",
"timestamp": 1659055936000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
3,
3
]
],
"date-time": "2024-03-03T12:30:45Z",
"timestamp": 1709469045799
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2021,
1,
9
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
9
]
],
"date-time": "2021-01-09T00:00:00Z",
"timestamp": 1610150400000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-139942/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-139942/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
1,
9
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2021,
1,
9
]
]
},
"publisher": "Research Square Platform LLC",
"reference-count": 0,
"references-count": 0,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.1186/s40001-021-00490-1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-139942/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Safety and Effectiveness of High-Dose Vitamin C in Patients with COVID-19; A Randomized Controlled open-label Clinical Trial ",
"type": "posted-content"
}