Pharmacokinetic Study of Antiviral Drugs in Patients with COVID-19
, K., Yakugaku Zasshi, doi:10.1248/yakushi.22-00169-1, Mar 2023
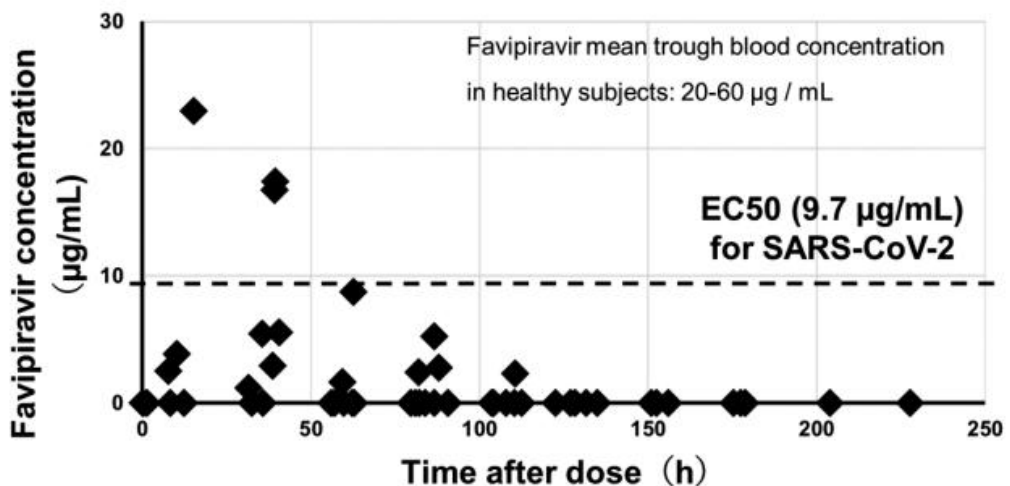
Pharmacokinetics study measuring the blood concentration of favipiravir in 7 critical patients in Japan, showing concentrations below the EC50 in 33 of 36 samples when using the standard dosing regimen. Authors note that patient characteristics significantly affect concentrations - there is higher clearance with increased body surface area and with the use of ventilation.
Irie et al., 1 Mar 2023, Japan, peer-reviewed, 1 author.
Contact: kei_irie@pharm.kobegakuin.ac.jp.
-Symposium Review -COVID-19 患者における抗ウイルス薬の薬物動態研究
The Faculty of Pharmaceutical Sciences, Kobe Gakuin University has collaborated in clinical research with Kobe City Medical Center General Hospital. In this university-medical institution collaboration, university faculty members discuss clinical problems with on-site pharmacists and doctors, and carry out clinical research to resolve these problems. During the coronavirus disease 2019 (COVID-19) pandemic, many patients with COVID-19 were treated at Kobe City Medical Center General Hospital. In February 2020, during the first increase in the number of patients with COVID-19 in Japan, treatment for COVID-19 was not established, and some existing anti-viral drugs, such as favipiravir, were experimentally used for COVID-19 treatment. However, since these drugs were not developed specifically for treating COVID-19, their pharmacokinetics have not been sufficiently studied. In particular, the pharmacokinetics of favipiravir in critically ill patients with COVID-19 was of concern, because critically ill patients have an urgent need for life-saving anti-viral drug treatment. Therefore, we conducted a collaborative clinical study to evaluate the pharmacokinetics of favipiravir in patients with COVID-19. The blood concentration of favipiravir in patients with COVID-19 at Kobe City Medical Center General Hospital was measured by lipid chromatography-tandem mass spectrometry at Kobe Gakuin University. Population pharmacokinetics analysis was then performed. In this symposium review, we introduce our pharmacokinetic study of antiviral drugs in patients with COVID-19, focusing on the university-medical institution collaboration. We believe collaborative clinical research will be useful for solving clinical issues and ensuring the effectiveness and safety of pharmacotherapies.
References
Bernal, Gomes Da Silva, Musungaie, Kovalchuk, Gonzalez et al., None, N. Engl. J. Med, doi:10.1056/NEJMoa2116044
Doi, Hibino, Hase, Yamamoto, Kasamatsu et al., None, Antimicrob. Agents Chemother, doi:10.1128/AAC.01897-20
Furuta, Komeno, Nakamura, None, Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci, doi:10.2183/pjab.93.027
Irie, Nakagawa, Fujita, Tamura, Eto et al., None, CPT Pharmacometrics Syst. Pharmacol, doi:10.1002/psp4.12685
Irie, Nakagawa, Fujita, Tamura, Eto et al., None, Clin. Transl. Sci
DOI record:
{
"DOI": "10.1248/yakushi.22-00169-1",
"ISSN": [
"0031-6903",
"1347-5231"
],
"URL": "http://dx.doi.org/10.1248/yakushi.22-00169-1",
"article-number": "22-00169-1",
"author": [
{
"affiliation": [
{
"name": "Faculty of Pharmaceutical Science, Kobe Gakuin University"
}
],
"family": "Irie",
"given": "Kei",
"sequence": "first"
}
],
"container-title": "YAKUGAKU ZASSHI",
"container-title-short": "YAKUGAKU ZASSHI",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
2,
28
]
],
"date-time": "2023-02-28T22:13:24Z",
"timestamp": 1677622404000
},
"deposited": {
"date-parts": [
[
2023,
3,
4
]
],
"date-time": "2023-03-04T03:31:44Z",
"timestamp": 1677900704000
},
"indexed": {
"date-parts": [
[
2023,
3,
4
]
],
"date-time": "2023-03-04T04:10:41Z",
"timestamp": 1677903041630
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2023,
3,
1
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.jstage.jst.go.jp/article/yakushi/143/3/143_22-00169-1/_pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "163",
"original-title": [],
"page": "239-241",
"prefix": "10.1248",
"published": {
"date-parts": [
[
2023,
3,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
3,
1
]
]
},
"publisher": "Pharmaceutical Society of Japan",
"reference": [
{
"DOI": "10.2183/pjab.93.027",
"doi-asserted-by": "crossref",
"key": "1",
"unstructured": "1) Furuta Y., Komeno T., Nakamura T., <i>Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci.</i>, <b>93</b>, 449–463 (2017)."
},
{
"key": "2",
"unstructured": "2) Irie K., Nakagawa A., Fujita H., Tamura R., Eto M., Ikesue H., Muroi N., Tomii K., Hashida T., <i>Clin. Transl. Sci.</i>, <b>13</b>, 880–885 (2020)."
},
{
"DOI": "10.1002/psp4.12685",
"doi-asserted-by": "crossref",
"key": "3",
"unstructured": "3) Irie K., Nakagawa A., Fujita H., Tamura R., Eto M., Ikesue H., Muroi N., Fukushima S., Tomii K., Hashida T., <i>CPT Pharmacometrics Syst. Pharmacol.</i>, <b>10</b>, 1161–1170 (2021)."
},
{
"DOI": "10.1128/AAC.01897-20",
"doi-asserted-by": "crossref",
"key": "4",
"unstructured": "4) Doi Y., Hibino M., Hase R., Yamamoto M., Kasamatsu Y., Hirose M., Mutoh Y., Homma Y., Terada M., Ogawa T., Kashizaki F., Yokoyama T., Koba H., Kasahara H., Yokota K., Kato H., Yoshida J., Kita T., Kato Y., Kamio T., Kodama N., Uchida Y., Ikeda N., Shinoda M., Nakagawa A., Nakatsumi H., Horiguchi T., Iwata M., Matsuyama A., Banno S., Koseki T., Teramachi M., Miyata M., Tajima S., Maeki T., Nakayama E., Taniguchi S., Lim C. K., Saijo M., Imai T., Yoshida H., Kabata D., Shintani A., Yuzawa Y., Kondo M., <i>Antimicrob. Agents Chemother.</i>, <b>64</b>, e01897–e20 (2020)."
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "crossref",
"key": "5",
"unstructured": "5) Jayk Bernal A., Gomes da Silva M. M., Musungaie D. B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M. L., Du J., Pedley A., Assaid C., Strizki J., Grobler J. A., Shamsuddin H. H., Tipping R., Wan H., Paschke A., Butterton J. R., Johnson M. G., De Anda C., <i>N. Engl. J. Med.</i>, <b>386</b>, 509–520 (2022)."
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.jstage.jst.go.jp/article/yakushi/143/3/143_22-00169-1/_article/-char/ja/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmaceutical Science",
"Pharmacology"
],
"subtitle": [],
"title": "Pharmacokinetic Study of Antiviral Drugs in Patients with COVID-19",
"type": "journal-article",
"volume": "143"
}