Oral Colchicine and Low-Dose Aspirin Combination Therapy for Non-elderly, Non-severe, Early Time From Onset, Adult Outpatients with Coronavirus Disease 2019 (COVID-19) during “The Fifth Pandemic Wave” in Japan
et al., The Kurume Medical Journal, doi:10.2739/kurumemedj.MS7012003, Mar 2024
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
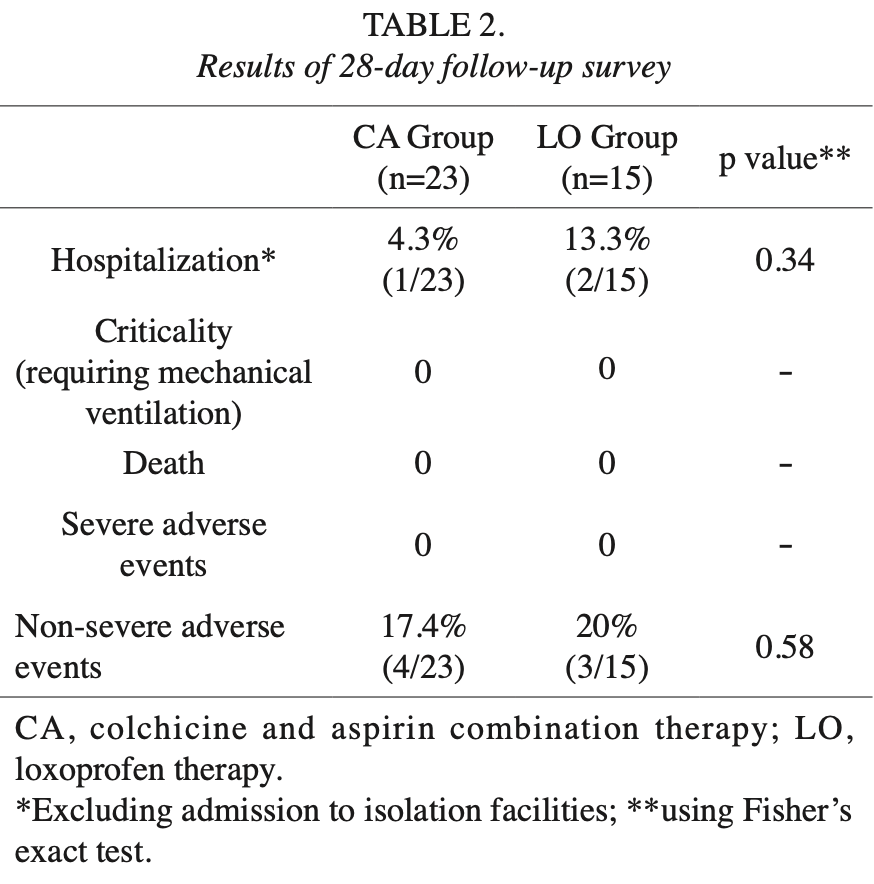
RCT 38 low risk outpatients in Japan, showing no significant differences for colchicine and low-dose aspirin compared to loxoprofen. Hospitalization was lower, without statistical significance (4.3% vs. 13.3%, p=0.34). There were no critical cases, deaths, or severe adverse events in either group.
Colchicine: 1.0mg loading dose, followed approximately half a day later by 0.5mg twice daily for 10 doses, and then 0.5 mg once daily for four doses. Aspirin: 100mg daily for 10 days. Both groups received probiotics and acetaminophen.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers aspirin and colchicine.
|
risk of hospitalization, 67.4% lower, RR 0.33, p = 0.55, treatment 1 of 23 (4.3%), control 2 of 15 (13.3%), NNT 11, day 28.
|
|
prolonged symptoms, 23.8% lower, RR 0.76, p = 0.72, treatment 8 of 21 (38.1%), control 6 of 12 (50.0%), NNT 8.4.
|
|
days until ≤37°C, 17.0% higher, relative time 1.17, p = 0.60, treatment 21, control 12.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Inokuchi et al., 19 Mar 2024, Randomized Controlled Trial, Japan, peer-reviewed, 16 authors, study period 27 July, 2021 - 6 September, 2021, average treatment delay 1.8 days, this trial compares with another treatment - results may be better when compared to placebo, this trial uses multiple treatments in the treatment arm (combined with aspirin) - results of individual treatments may vary.
Contact: kita-sir1028@jcom.home.ne.jp.
Oral Colchicine and Low-Dose Aspirin Combination Therapy for Nonelderly, Non-severe, Early Time From Onset, Adult Outpatients with Coronavirus Disease 2019 (COVID-19) during "The Fifth Pandemic Wave" in Japan
Background: Treatment with antiviral drugs for non-severe, early time from onset, adult outpatients with Coronavirus Disease 2019 (COVID-19) had not been established in 2021. However, some new variants of SARS-CoV-2 had caused rapid exacerbation and hospitalization among non-elderly outpatients with COVID-19, contributing to widespread crises within healthcare systems. Methods: From July to October 2021, we urgently assessed a therapeutic program using oral colchicine (1.0 mg loading dose, followed approximately half a day later by 0.5 mg twice daily for 5 days, and then 0.5 mg once daily for 4 days) and low-dose aspirin (100 mg once daily for 10 days), for non-elderly, non-severe, early time from onset, adult outpatients with COVID-19. To verify its effectiveness, we set loxoprofen as a control arm, and comparison of these two arms was performed. The primary outcomes were hospitalization, criticality, and death rates. Results: Thirty-eight patients (23 receiving colchicine and low-dose aspirin [CA]; 15 receiving loxoprofen [LO]) were evaluated. Hospitalization rate was lower in the CA group (1/23; 4.3%) than in the LO group (2/15; 13.3%); however, no significant difference was found between the two groups (p=0.34). No critical cases, deaths, or severe adverse events were found in either group. Conclusions: Our CA regimen did not show superiority over LO treatment. However, our clinical experience should be recorded as part of community health care activities carried out in Kurume City against the unprecedented COVID-19 pandemic.
References
Absalón-Aguilar, Rull-Gabayet, Pérez-Fragoso, Mejía-Domínguez, Núñez-Álvarez, Colchicine is safe though ineffective in the treatment of severe COVID-19: a randomized clinical trial (COLCHIVID), J Gen Intern Med
Agarwal, Rochwerg, Lamontagne, Siemieniuk, Agoritsas, Update to living WHO guideline on drugs for COVID-19, BMJ
Aleem, Samad, Slenker, Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19)
Brunetti, Diawara, Tsai, Firestein, Nahass, Colchicine to weather the cytokine storm in hospitalized patients with COVID-19, J Clin Med
Chow, Khanna, Kethireddy, Yamane, Levine, Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019, Anesth Analg
Connors, Brooks, Sciurba, Krishnan, Bledsoe, Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial, JAMA
Davies, Abbott, Barnard, Jarvis, Kucharski, Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England, Science
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019; the GRECCO-19 randomized clinical trial, JAMA COLCHICINE AND ASPIRIN FOR COVID-19 Netw Open
Downing, Chauhan, Chaudry, Galwankar, Sharma, Colchicine, aspirin, and montelukast -a case of successful combined pharmacotherapy for adult multisystem inflammatory syndrome in COVID-19, J Glob Infect Dis
Fisman, Tuite, Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada, CMAJ
Gao, Guo, Luo, Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert!, J Med Virol
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya, Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Kalil, Patterson, Mehta, Tomashek, Wolfe, Baricitinib plus remdesivir for hospitalized adults with COVID-19, N Engl J Med
Kevorkian, Lopes, Sène, Riveline, Vandiedonck, Oral corticoid, aspirin, anticoagulant, colchicine, and furosemide to improve the outcome of hospitalized COVID-19 patients -the COCAA-COLA cohort study, J Infect
Khoo, Fitzgerald, Fletcher, Ewings, Jaki, Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study, J Antimicrob Chemother
Kitasato, Imaoka, Shinotsuka, Miyamoto, Ishikawa, Our clinical experience of using colchicine against the COVID-19 "4th epidemic wave" in Japan, Rinsho to Kenkyu
Mahase, COVID-19: Molnupiravir reduces risk of hospi-tal admission or death by 50% in patients at risk, MSD reports, BMJ
Mahase, COVID-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ
Mehta, Patel, Chavda, Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials, RMD Open
Mikolajewska, Fischer, Piechotta, Mueller, Metzendorf, Colchicine for the treatment of COVID-19, Cochrane Database Syst Rev
Mukae, Yotsuyanagi, Ohmagari, Doi, Imamura, Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019: The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study, Clin Infect Dis
Razonable, Pawlowski, Horo, Arndt, Arndt, Casirivimab-imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, EClinicalMedicine
Recovery Collaborative, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Recovery Collaborative, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet Respir Med
Salah, Mehta, Meta-analysis of the effect of colchicine on mortality and mechanical ventilation in COVID-19, Am J Cardiol
Sheikh, Mcmenamin, Taylor, Robertson, Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness, Lancet
Suzuki, Yamasoba, Kimura, Wang, Kishimoto, Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant, Nature
Tani-Sassa, Iwasaki, Ichimura, Nagano, Takatsuki, Viral loads and profile of the patients infected with SARS-CoV-2 Delta, Alpha, or R.1 variants in Tokyo, J Med Virol
Tardif, Bouabdallaoui, Allier, Gaudet, Shah, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled multicentre trial, Lancet Respir Med
Thachil, Tang, Gando, Falanga, Cattaneo, ISTH interim guidance on recognition and management of coagulopathy in COVID-19, J Thromb Haemost
The, Group, Horby, Lim, Emberson et al., Dexamethasone in hospitalized patients with COVID-19, N Engl J Med
Thrupthi, Ganti, Acherjee, Mehmood, Vakde, A rare case of acute pericarditis due to SARS-CoV-2 managed with aspirin and colchicine, Cureus
Toyoda, Yasaka, Uchiyama, Nagao, Gotoh, Blood pressure levels and bleeding events during antithrombotic therapy: the Bleeding with Antithrombotic Therapy (BAT) Study, Stroke
Van Doremalen, Bushmaker, Morris, Holbrook, Gamble, Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1, N Engl J Med
Weinreich, Sivapalasingam, Norton, Ali, Gao, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Wong, Lau, Au, Xiong, Chung, Optimal timing of remdesivir initiation in hospitalized COVID-19 patients administered with dexamethasone, Clin Infect Dis
DOI record:
{
"DOI": "10.2739/kurumemedj.ms7012003",
"ISSN": [
"0023-5679",
"1881-2090"
],
"URL": "http://dx.doi.org/10.2739/kurumemedj.MS7012003",
"article-number": "MS7012003",
"author": [
{
"affiliation": [
{
"name": "Kurume Physicians Association (Kurume Naikaikai)"
}
],
"family": "INOKUCHI",
"given": "TETSUAKI",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Homma Cardiovascular Clinic"
}
],
"family": "HOMMA",
"given": "TOMOKI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kurume Physicians Association (Kurume Naikaikai)"
}
],
"family": "KITASATO",
"given": "YASUHIKO",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "AKIYAMA",
"given": "MAYU",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "CHIKASUE",
"given": "AYAKO",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "NISHII",
"given": "YUUYA",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kurume Physicians Association (Kurume Naikaikai)"
}
],
"family": "BAN",
"given": "SHIGEKI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kurume Physicians Association (Kurume Naikaikai)"
}
],
"family": "ADACHI",
"given": "TAKEKI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "SONEZAKI",
"given": "AYA",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "MASUDA",
"given": "HIROSHI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "KAMEI",
"given": "HIDEKI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "TAKENAKA",
"given": "MIKI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "JCHO Kurume General Hospital"
}
],
"family": "TANAKA",
"given": "MAKI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respirology, NHO Kyushu Medical Center"
}
],
"family": "OKAMOTO",
"given": "MASAKI",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respirology, Neurology, and Rheumatology, Department of Medicine, Kurume University School of Medicine"
}
],
"family": "HOSHINO",
"given": "TOMOAKI",
"sequence": "additional"
},
{
"affiliation": [],
"name": "THE K-COCOA (KURUME-COVID-19 THERAPEUTIC PROGRAM BY COLCHICINE AND LOW-DOSE ASPIRIN) STUDY COLLABORATORS",
"sequence": "additional"
}
],
"container-title": "The Kurume Medical Journal",
"container-title-short": "Kurume Med. J.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
3,
18
]
],
"date-time": "2024-03-18T22:13:13Z",
"timestamp": 1710799993000
},
"deposited": {
"date-parts": [
[
2024,
3,
18
]
],
"date-time": "2024-03-18T22:13:13Z",
"timestamp": 1710799993000
},
"indexed": {
"date-parts": [
[
2024,
3,
19
]
],
"date-time": "2024-03-19T00:26:41Z",
"timestamp": 1710808001328
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3,
19
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.jstage.jst.go.jp/article/kurumemedj/advpub/0/advpub_MS7012003/_pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1445",
"original-title": [],
"prefix": "10.2739",
"published": {
"date-parts": [
[
2024,
3,
19
]
]
},
"published-print": {
"date-parts": [
[
2024,
3,
19
]
]
},
"publisher": "Kurume Medical Journal",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.jstage.jst.go.jp/article/kurumemedj/advpub/0/advpub_MS7012003/_article"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Oral Colchicine and Low-Dose Aspirin Combination Therapy for Non-elderly, Non-severe, Early Time From Onset, Adult Outpatients with Coronavirus Disease 2019 (COVID-19) during “The Fifth Pandemic Wave” in Japan",
"type": "journal-article"
}
