Nigella sativa oil (NSO) as an adjuvant in the management of mild COVID-19 infection in Kaduna state
et al., The Nigerian Health Journal, doi:10.60787/tnhj-712, Jan 2024
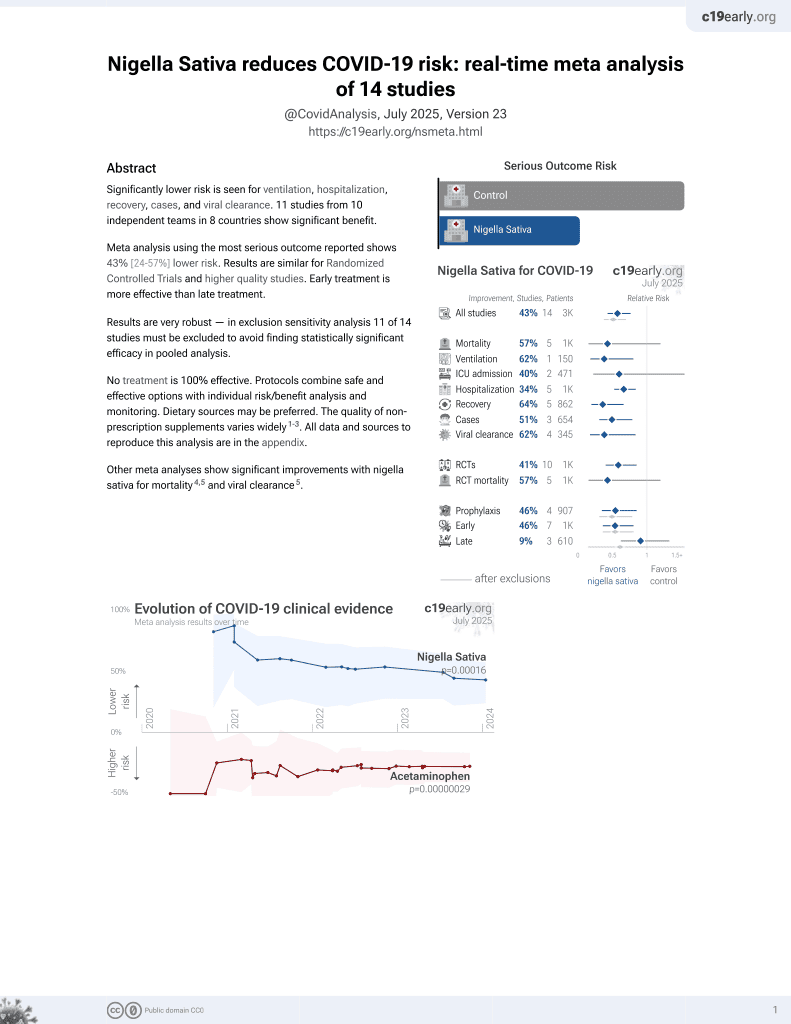
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study of 51 mild COVID-19 cases in Nigeria, showing faster recovery and improved viral clearance with nigella sativa oil (NSO) treatment. NSO patients received 5mL twice daily in addition to usual care (zinc, vitamin C and a multivitamin).
|
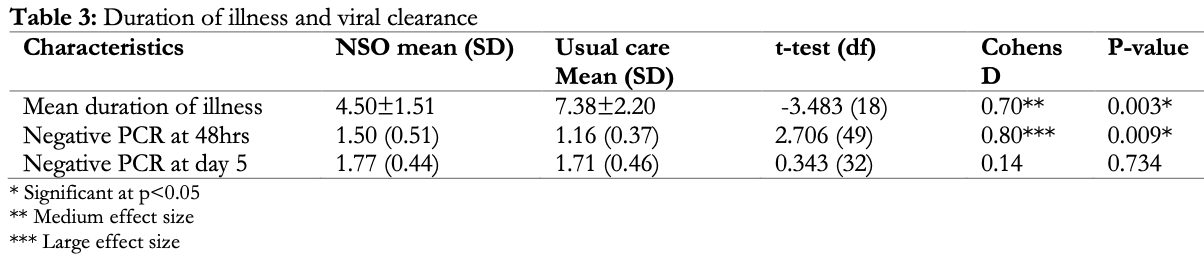
recovery time, 39.0% lower, relative time 0.61, p = 0.003, treatment mean 4.5 (±1.51) n=26, control mean 7.38 (±2.2) n=25.
|
|
risk of no viral clearance, 15.4% lower, RR 0.85, p = 1.00, treatment 3 of 13 (23.1%), control 6 of 22 (27.3%), NNT 24, day 5.
|
|
risk of no viral clearance, 40.5% lower, RR 0.60, p = 0.02, treatment 13 of 26 (50.0%), control 21 of 25 (84.0%), NNT 2.9, day 2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Idris et al., 15 Jan 2024, prospective, Nigeria, peer-reviewed, mean age 30.8, 8 authors, study period 27 October, 2020 - 20 May, 2021.
Contact: twinsbrother17@gmail.com.
Nigella sativa oil (NSO) as an adjuvant in the management of mild COVID-19 infection in Kaduna state
doi:10.60787/tnhj-712
Background: To assess the efficacy of Nigella sativa oil (NSO) in the management of mild COVID-19 infection in Kaduna state. Method: Quasi-experimental study among 51 mild COVID-19 cases enrolled in Hamdala isolation center from 27 th October, 2020 to 20 th May, 2021. Outcome variables were viral clearance, resolution of symptoms and duration of hospital stay after commencement of the different treatment regimen at level of significance P < 0.05 and effect size (Cohen's D 0.2= small, 0.5=medium and ≥0.8 = large). Result: Out of the 51 people enrolled in the study, 26 (51%) were placed on NSO plus usual care while 25 (49%) were on usual care alone with Mean age (SD) of 30.77±14.56 and 32.60±17.50 respectively. There were 16 (61.5%) females and 10 (38.5%) males in the NSO group and 19 (76%) females with 6 (24%) males in the usual care group. More patients on NSO have symptoms 12 (46.2%); ranging from fever, malaise, anosmia and loss of taste compared to 8 (32.0%) of the usual care group. Mean recovery time was significantly shorter 4.50±1.51 days in the NSO group, compared to 7.38±2.20 in the usual care with medium effect size (t-value = -3.483, Cohen's D = 0.7, P = 0.003). Repeat PCR test was significantly different 48hours after commencement of treatment between groups, with large effect size (t=2.706, Cohen's D=0.8, p=0.009). Conclusion: NSO as add-on therapeutic agent was associated with faster recovery, viral clearance and shorter duration of care than usual care alone in patients with mild COVID-19 infection.
References
Ahmad, The race to treat COVID-19: Potential therapeutic agents for the prevention and treatment of SARS-CoV-2, European journal of medicinal chemistry
Ashraf, Ashraf, Ashraf, Imran, Kalsoom et al., Therapeutic efficacy of Honey and Nigella sativa against COVID-19: A multicenter randomized controlled clinical trial (HNS-COVID-PK), medRxiv
Bk, Immunomodulatory effects of black seeds and garlic on alloxan-induced diabetes in albino rat, Allergologia et immunopathologia
Boskabady, Mohsenpoor, Takaloo, Antiasthmatic effect of Nigella sativa in airways of asthmatic patients, Phytomedicine
Cao, Zhang, Wang, Lu, Zhu et al., Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China
Covid, outbreak in Nigeria situation report-Abuja: Nigeria centre for disease control
Eğilmez, Kökten, Kalcıoğlu, Ekici, Şerifler et al., Investigation of the protective effect of Nigella sativa oil in cisplatin induced oral mucositis: an experimental study, Turkish archives of otorhinolaryngology
He, Guo, Mao, Zhang, Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta-analysis, Journal of medical virology
Idris, Moses, Muhammad, Arinola, Muazu et al., Socio-Demographic and Clinical Characteristics and Outcomes of COVID-19 Patients in Kaduna State, Journal of Biochemistry, Microbiology and Biotechnology
Idris, Oyefabi, Dalhat, Umar, Abdulmajid et al., Treatment Outcomes in COVID-19 patients with comorbidities in Kaduna state, Northwestern Nigeria, Nigerian Health Journal
Imran, Khan, Abida, Alshammari, Alkhaldi et al., Nigella sativa L. and COVID-19: A glance at the anti-COVID-19 chemical constituents, clinical trials, inventions, and patent literature, Molecules
Ka, Gastroprotective effects of Nigella Sativa oil on the formation of stress gastritis in hypothyroidal rats, International Journal of Physiology, Pathophysiology and Pharmacology
Koshak, Koshak, Mobeireek, Badawi, Wali et al., Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial, Complementary Therapies in Medicine
Lin, Hsu, Lin, Antiviral natural products and herbal medicines, Journal of traditional and complementary medicine
Osibogun, Balogun, Abayomi, Idris, Kuyinu et al., Outcomes of COVID-19 patients with comorbidities in southwest Nigeria, PloS one
Oyefabi, Musa, Kambai, Usman, Gwamna et al., Comparison of the Ivermectin and Lopinavir/Ritonavir Treatment Outcomes among COVID-19 Mild to Moderate Cases in Kaduna State, West African Journal of Medicine
Rocha-Filho, Albuquerque, Carvalho, Pereira Gama, Magalhães, Headache, anosmia, ageusia and other neurological symptoms in COVID-19: a crosssectional study, The Journal of Headache and Pain
Rodriguez-Guerra, Jadhav, Vittorio, Current treatment in COVID-19 disease: a rapid review, Drugs in Context
Shirvani, Rostamkhani, Arabzadeh, Mohammadi, Mohammadi, Potential role of Nigella sativa supplementation with physical activity in prophylaxis and treatment of COVID-19: a contemporary review, Sport Sciences for Health
Singh, De, Antiviral agents for the treatment of COVID-19: Progress and challenges, Cell Reports Medicine
Stawicki, Jeanmonod, Miller, Paladino, Gaieski et al., The 2019-2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: A joint american college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper, Journal of global infectious diseases
Tavakkoli, Mahdian, Razavi, Hosseinzadeh, Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone, Journal of pharmacopuncture
DOI record:
{
"DOI": "10.60787/TNHJ-712",
"URL": "https://tnhjph.com/index.php/tnhj/article/view/712",
"author": [
{
"family": "Idris",
"given": "U"
},
{
"family": "Abdulmajid",
"given": "M"
},
{
"family": "Umar",
"given": "IM"
},
{
"family": "Oyefabi",
"given": "AM"
},
{
"family": "Musa",
"given": "AN"
},
{
"family": "Ogunsina",
"given": "AM"
},
{
"family": "Abdu-Aguye",
"given": "I"
},
{
"family": "Olasinde",
"given": "TA"
}
],
"container-title": "The Nigerian Health Journal",
"id": "https://doi.org/10.60787/tnhj-712",
"issued": {
"date-parts": [
[
2023
]
]
},
"publisher": "The Nigerian Health Journal",
"title": "Nigella sativa oil (NSO) as an adjuvant in the management of mild COVID-19 infection in Kaduna state",
"type": "article-journal"
}