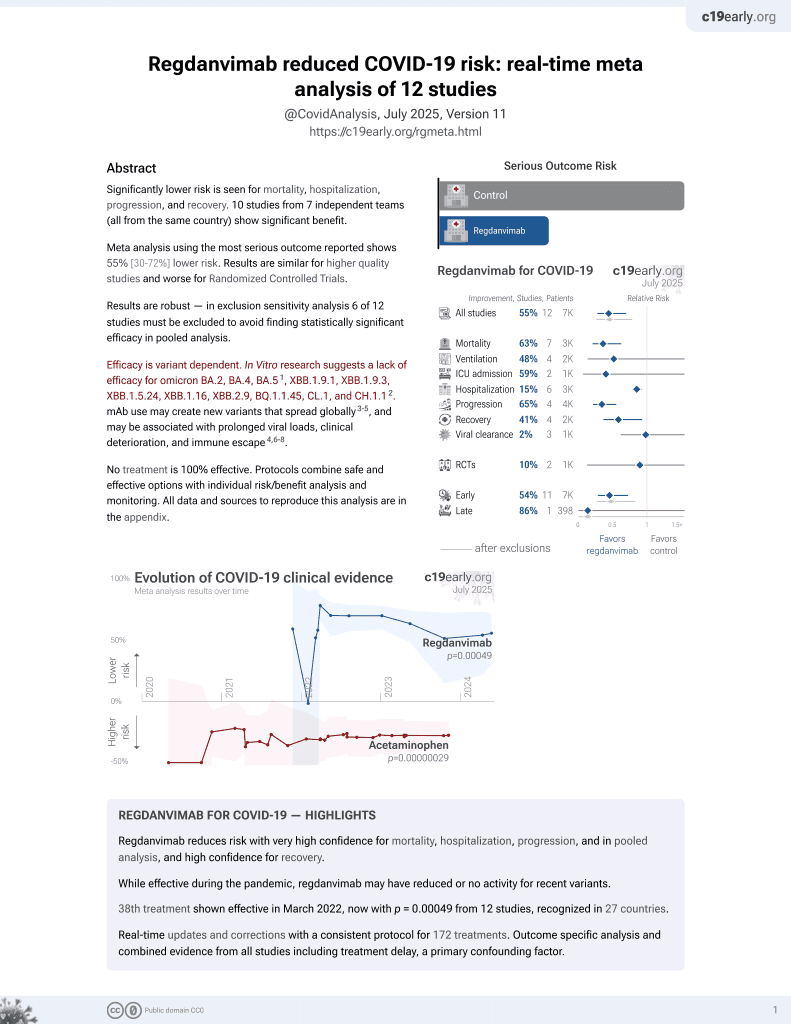
Effect of Regdanvimab on Mortality in Patients Infected with SARS-CoV-2 Delta Variants: A Propensity Score-Matched Cohort Study
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-024-00971-w, Apr 2024
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
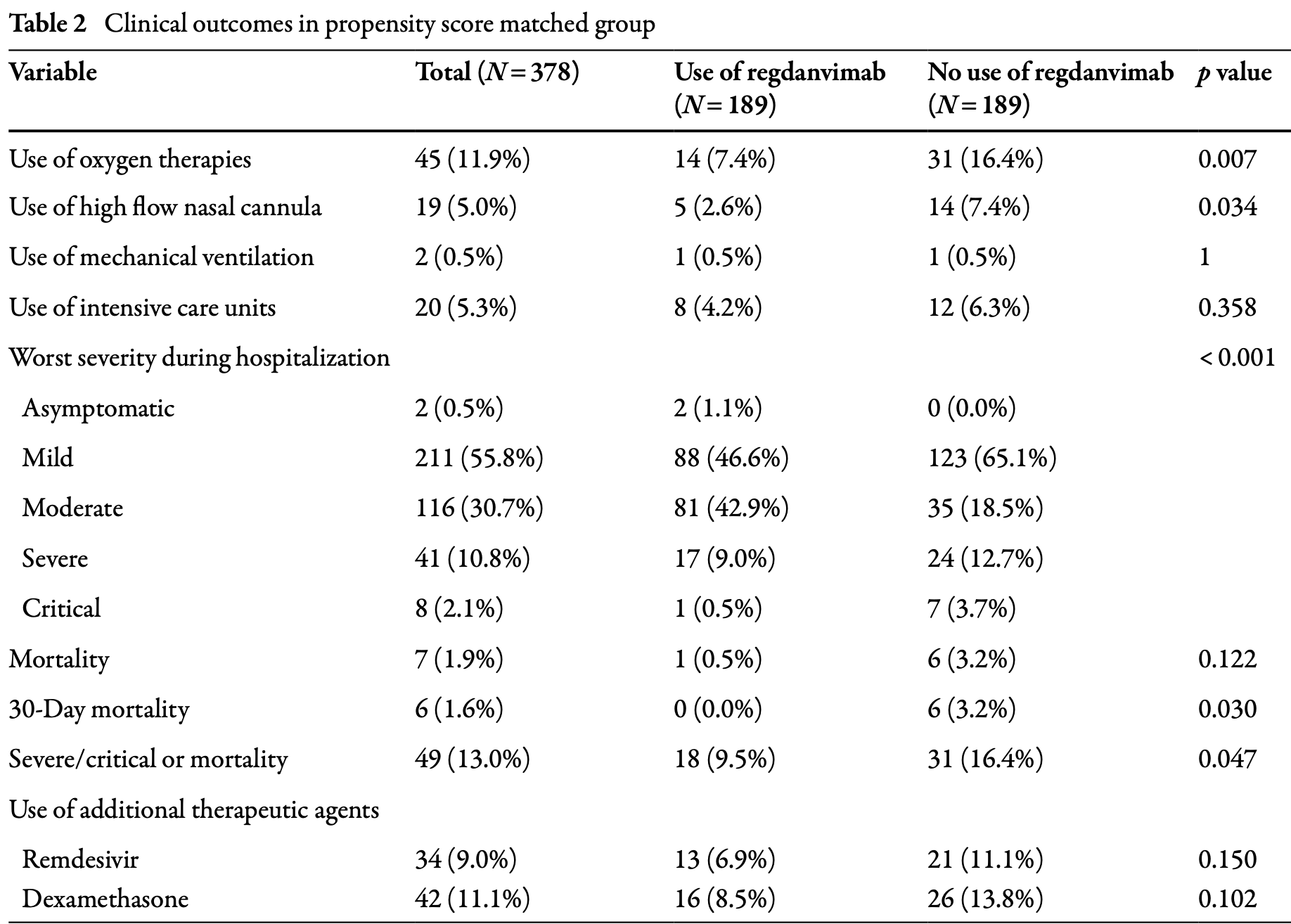
PSM retrospective 378 hospitalized COVID-19 patients in Korea showing lower progression with regdanvimab treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
|
risk of death, 83.3% lower, RR 0.17, p = 0.12, treatment 1 of 189 (0.5%), control 6 of 189 (3.2%), NNT 38.
|
|
risk of death, 92.3% lower, RR 0.08, p = 0.03, treatment 0 of 189 (0.0%), control 6 of 189 (3.2%), NNT 32, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 30.
|
|
risk of mechanical ventilation, no change, RR 1.00, p = 1.00, treatment 1 of 189 (0.5%), control 1 of 189 (0.5%).
|
|
risk of ICU admission, 33.3% lower, RR 0.67, p = 0.49, treatment 8 of 189 (4.2%), control 12 of 189 (6.3%), NNT 47.
|
|
risk of oxygen therapy, 54.8% lower, RR 0.45, p = 0.01, treatment 14 of 189 (7.4%), control 31 of 189 (16.4%), NNT 11.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hwang et al., 12 Apr 2024, retrospective, South Korea, peer-reviewed, 13 authors, study period 26 May, 2021 - 30 January, 2022.
Contact: ktkwon@knu.ac.kr, changhee@gnu.ac.kr.
Effect of Regdanvimab on Mortality in Patients Infected with SARS-CoV-2 Delta Variants: A Propensity Score-Matched Cohort Study
Infectious Diseases and Therapy, doi:10.1007/s40121-024-00971-w
Introduction: Regdanvimab, a monoclonal antibody pharmaceutical, is the first Korean drug approved for treating coronavirus disease 2019 . We analyzed the therapeutic efficacy of regdanvimab in patients with the COVID-19 delta variant infection.
Methods: We retrospectively reviewed the electronic medical records of patients hospitalized at two Korean tertiary COVID-19 hospitals with COVID-19 delta variant infection between May 26, 2021, and January 30, 2022. To analyze the therapeutic efficacy of regdanvimab, the patients were divided into regdanvimab and non-regdanvimab groups and were 1:1 propensity-score (PS)-matched on age, severity at admission, and COVID-19 vaccination history. Results: Of 492 patients, 262 (53.3%) and 230 (46.7%) were in the regdanvimab and non-regdanvimab groups, respectively. After PS matching the groups on age, severity at admission, and COVID-19 vaccination history, each group comprised 189 patients. The 30-day hospital
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aggarwal, Stella, Walker, SARS-CoV-2 Omicron: evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern, medRxiv
Alexandar, Ravisankar, Kumar, Jakkan, A comprehensive review on Covid-19 delta variant, Int J Pharmacol Clin Res (IJPCR)
Deb, Molla, Saif-Ur-Rahman, An update to monoclonal antibody as therapeutic option against COVID-19, Biosaf Health
Greaney, Starr, Gilchuk, Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition, Cell Host Microbe
Holland, Kellett, A systematic review of the discrimination and absolute mortality predicted by the National Early Warning Scores according to different cut-off values and prediction windows, Eur J Intern Med
Hurt, Wheatley, Neutralizing antibody therapeutics for COVID-19, Viruses
Jacobs, Haidar, Mellors, COVID-19: challenges of viral variants, Ann Rev Med
Jang, Oh, Kim, Regdanvimab for patients with mild-to-moderate COVID-19: a retrospective cohort study and subgroup analysis of patients with the delta variant, Int J Infect Dis
Khedar, Mittal, Ambaliya, Greater Covid-19 severity and mortality in hospitalized patients in second (delta variant) wave compared to the first: single centre prospective study in India, medRxiv
Kim, Jang, Lee, Effectiveness of regdanvimab treatment for SARS-CoV-2 delta variant, which exhibited decreased in vitro activity: a nationwide real-world multicenter cohort study, Front Cell Infect Microbiol
Kim, Ryu, Lee, A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat Commun
Kodera, Rashed, Hirata, Estimation of realworld vaccination effectiveness of mRNA COVID-19 vaccines against delta and omicron variants in Japan, Infect Dis Ther
Kwak, Song, Kang, Use of the monoclonal antibody regdanvimab to treat patients hospitalized with COVID-19: real-world data during the delta variant predominance, Infect Chemother
Lee, Park, Early oxygen requirement in patients with wild-to-moderate COVID-19 who received regdanvimab after delta-variant outbreak, Infect Chemother
O'horo, Challener, Speicher, Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant, Mayo Clin Proc
Park, Lim, Kim, Clinical and virological characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.617.2 (delta) variant: a prospective cohort study, Clin Infect Dis
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 omicron to antibody neutralization, Nature
Ryu, Kang, Noh, The in vitro and in vivo efficacy of CT-P59 against gamma, delta and its associated variants of SARS-CoV-2, Biochem Biophys Res Commun
Silcock, Corfield, Staines, Rooney, Superior performance of National Early Warning Score compared with quick Sepsis-related Organ Failure Assessment Score in predicting adverse outcomes: a retrospective observational study of patients in the prehospital setting, Eur J Emerg Med
Streinu-Cercel, Săndulescu, Preotescu, Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebocontrolled trial in outpatients with mild-to-moderate coronavirus disease 2019, Open Forum Infect Dis
Syed, Regdanvimab: First approval, Drugs
Tchang, Askin, Sahagun, The independent risk of obesity and diabetes and their interaction in COVID-19: a retrospective cohort study, Obesity
Tenforde, Self, Gaglani, Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death-United States, March 2021-January 2022, Morb Mortal Wkly Rep
Touret, Baronti, Bouzidi, De Lamballerie, In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B. 1.1. 529 529 isolate, Sci Rep
Twohig, Nyberg, Zaidi, Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B. 1.617. 2) compared with alpha (B. 1.1. 7) variants of concern: a cohort study, Lancet Infect Dis
Zali, Khodadoost, Gholamzadeh, Mortality among hospitalized COVID-19 patients during surges of SARS-CoV-2 alpha (B. 1.1. 7) and delta (B. 1. 617. 2) variants, Sci Rep
Zhao, Huang, Zhang, Chen, Gao et al., The global transmission of new coronavirus variants, Environ Res
DOI record:
{
"DOI": "10.1007/s40121-024-00971-w",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-024-00971-w",
"alternative-id": [
"971"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "15 February 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "12 April 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Soyoon Hwang, Nan Young Lee, Eunkyung Nam, Yu Kyung Kim, Shin-Woo Kim, Hyun-Ha Chang, Yoonjung Kim, Sohyun Bae, Juhwan Jeong, Jae-Ho Shin, Guehwan Jang, Changhee Lee, and Ki Tae Kwon declare no conflicts of interest."
},
{
"group": {
"label": "Ethical Approval and Informed Consent",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "This study was approved by the Daegu Joint Institutional Review Board, and the requirement for official written informed consent was waived (DGIRB 2021-10-002), given the retrospective nature of the study (using EMR and residual samples)."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3618-174X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hwang",
"given": "Soyoon",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-3381-1382",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Nan Young",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9845-3563",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nam",
"given": "Eunkyung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4699-8502",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Yu Kyung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3755-8249",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Shin-Woo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9405-2121",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chang",
"given": "Hyun-Ha",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7454-4014",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Yoonjung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0206-7108",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bae",
"given": "Sohyun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0003-5004-6998",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jeong",
"given": "Juhwan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6450-9787",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shin",
"given": "Jae-Ho",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3108-8087",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jang",
"given": "Guehwan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5930-5461",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Changhee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4666-0672",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kwon",
"given": "Ki Tae",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
12
]
],
"date-time": "2024-04-12T13:01:45Z",
"timestamp": 1712926905000
},
"deposited": {
"date-parts": [
[
2024,
4,
12
]
],
"date-time": "2024-04-12T13:31:27Z",
"timestamp": 1712928687000
},
"funder": [
{
"DOI": "10.13039/100018592",
"doi-asserted-by": "publisher",
"name": "Celltrion"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
13
]
],
"date-time": "2024-04-13T00:36:43Z",
"timestamp": 1712968603316
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
4,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
12
]
],
"date-time": "2024-04-12T00:00:00Z",
"timestamp": 1712880000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
12
]
],
"date-time": "2024-04-12T00:00:00Z",
"timestamp": 1712880000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-024-00971-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-024-00971-w/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-024-00971-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2024,
4,
12
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.bsheal.2021.02.001",
"author": "P Deb",
"doi-asserted-by": "publisher",
"first-page": "87",
"issue": "2",
"journal-title": "Biosaf Health",
"key": "971_CR1",
"unstructured": "Deb P, Molla MMA, Saif-Ur-Rahman K. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health. 2021;3(2):87–91.",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-20602-5",
"author": "C Kim",
"doi-asserted-by": "publisher",
"first-page": "288",
"issue": "1",
"journal-title": "Nat Commun",
"key": "971_CR2",
"unstructured": "Kim C, Ryu D-K, Lee J, et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021;12(1):288.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1007/s40265-021-01626-7",
"author": "YY Syed",
"doi-asserted-by": "publisher",
"first-page": "2133",
"issue": "18",
"journal-title": "Drugs",
"key": "971_CR3",
"unstructured": "Syed YY. Regdanvimab: First approval. Drugs. 2021;81(18):2133–7.",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofac053",
"author": "A Streinu-Cercel",
"doi-asserted-by": "publisher",
"first-page": "ofac053",
"journal-title": "Open Forum Infect Dis",
"key": "971_CR4",
"unstructured": "Streinu-Cercel A, Săndulescu O, Preotescu L-L, et al. Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019. Open Forum Infect Dis. 2022;9:ofac053.",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/j.envres.2021.112240",
"author": "Y Zhao",
"doi-asserted-by": "publisher",
"journal-title": "Environ Res",
"key": "971_CR5",
"unstructured": "Zhao Y, Huang J, Zhang L, Chen S, Gao J, Jiao H. The global transmission of new coronavirus variants. Environ Res. 2022;206: 112240.",
"volume": "206",
"year": "2022"
},
{
"DOI": "10.1146/annurev-med-042921-020956",
"author": "JL Jacobs",
"doi-asserted-by": "publisher",
"first-page": "31",
"journal-title": "Ann Rev Med",
"key": "971_CR6",
"unstructured": "Jacobs JL, Haidar G, Mellors JW. COVID-19: challenges of viral variants. Ann Rev Med. 2023;74:31–53.",
"volume": "74",
"year": "2023"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"author": "D Planas",
"doi-asserted-by": "publisher",
"first-page": "671",
"issue": "7898",
"journal-title": "Nature",
"key": "971_CR7",
"unstructured": "Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature. 2022;602(7898):671–5.",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1016/j.bbrc.2021.09.023",
"author": "D-K Ryu",
"doi-asserted-by": "publisher",
"first-page": "91",
"journal-title": "Biochem Biophys Res Commun",
"key": "971_CR8",
"unstructured": "Ryu D-K, Kang B, Noh H, et al. The in vitro and in vivo efficacy of CT-P59 against gamma, delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021;578:91–6.",
"volume": "578",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2022.12.035",
"author": "YR Jang",
"doi-asserted-by": "publisher",
"first-page": "94",
"journal-title": "Int J Infect Dis",
"key": "971_CR9",
"unstructured": "Jang YR, Oh YJ, Kim JY. Regdanvimab for patients with mild-to-moderate COVID-19: a retrospective cohort study and subgroup analysis of patients with the delta variant. Int J Infect Dis. 2023;130:94–100.",
"volume": "130",
"year": "2023"
},
{
"DOI": "10.3947/ic.2022.0103",
"author": "YG Kwak",
"doi-asserted-by": "publisher",
"first-page": "781",
"issue": "4",
"journal-title": "Infect Chemother",
"key": "971_CR10",
"unstructured": "Kwak YG, Song JE, Kang J, et al. Use of the monoclonal antibody regdanvimab to treat patients hospitalized with COVID-19: real-world data during the delta variant predominance. Infect Chemother. 2022;54(4):781.",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.3389/fcimb.2023.1192512",
"author": "H Kim",
"doi-asserted-by": "publisher",
"first-page": "1192512",
"journal-title": "Front Cell Infect Microbiol",
"key": "971_CR11",
"unstructured": "Kim H, Jang YR, Lee JY, et al. Effectiveness of regdanvimab treatment for SARS-CoV-2 delta variant, which exhibited decreased in vitro activity: a nationwide real-world multicenter cohort study. Front Cell Infect Microbiol. 2023;13:1192512.",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7112e1",
"author": "MW Tenforde",
"doi-asserted-by": "publisher",
"first-page": "459",
"issue": "12",
"journal-title": "Morb Mortal Wkly Rep",
"key": "971_CR12",
"unstructured": "Tenforde MW, Self WH, Gaglani M, et al. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. Morb Mortal Wkly Rep. 2022;71(12):459.",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.3390/vaccines10030430",
"author": "S Kodera",
"doi-asserted-by": "publisher",
"first-page": "430",
"issue": "3",
"journal-title": "Vaccines",
"key": "971_CR13",
"unstructured": "Kodera S, Rashed EA, Hirata A. Estimation of real-world vaccination effectiveness of mRNA COVID-19 vaccines against delta and omicron variants in Japan. Vaccines. 2022;10(3):430.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.mayocp.2021.12.002",
"author": "JC O’Horo",
"doi-asserted-by": "publisher",
"first-page": "327",
"journal-title": "Mayo Clin Proc",
"key": "971_CR14",
"unstructured": "O’Horo JC, Challener DW, Speicher L, et al. Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant. Mayo Clin Proc. 2022;97:327–32.",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac239",
"author": "S Park",
"doi-asserted-by": "publisher",
"first-page": "e27",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "971_CR15",
"unstructured": "Park S, Lim SY, Kim JY, et al. Clinical and virological characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.617.2 (delta) variant: a prospective cohort study. Clin Infect Dis. 2022;75(1):e27–34.",
"volume": "75",
"year": "2022"
},
{
"key": "971_CR16",
"unstructured": "National Institutes of Health. National Institutes of Health COVID-19 treatment guidelines. Therapeutic management of nonhospitalized adults with COVID. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/. Accessed 2 Nov 2023."
},
{
"DOI": "10.3947/ic.2022.0011",
"author": "CM Lee",
"doi-asserted-by": "publisher",
"first-page": "258",
"issue": "2",
"journal-title": "Infect Chemother",
"key": "971_CR17",
"unstructured": "Lee CM, Park S-W, Lee E. Early oxygen requirement in patients with wild-to-moderate COVID-19 who received regdanvimab after delta-variant outbreak. Infect Chemother. 2022;54(2):258.",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1002/oby.23172",
"author": "BG Tchang",
"doi-asserted-by": "publisher",
"first-page": "971",
"issue": "6",
"journal-title": "Obesity",
"key": "971_CR18",
"unstructured": "Tchang BG, Askin G, Sahagun A, et al. The independent risk of obesity and diabetes and their interaction in COVID-19: a retrospective cohort study. Obesity. 2021;29(6):971–5.",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/j.ejim.2021.12.024",
"author": "M Holland",
"doi-asserted-by": "publisher",
"first-page": "15",
"journal-title": "Eur J Intern Med",
"key": "971_CR19",
"unstructured": "Holland M, Kellett J. A systematic review of the discrimination and absolute mortality predicted by the National Early Warning Scores according to different cut-off values and prediction windows. Eur J Intern Med. 2022;98:15–26.",
"volume": "98",
"year": "2022"
},
{
"DOI": "10.1097/MEJ.0000000000000589",
"author": "DJ Silcock",
"doi-asserted-by": "publisher",
"first-page": "433",
"issue": "6",
"journal-title": "Eur J Emerg Med",
"key": "971_CR20",
"unstructured": "Silcock DJ, Corfield AR, Staines H, Rooney KD. Superior performance of National Early Warning Score compared with quick Sepsis-related Organ Failure Assessment Score in predicting adverse outcomes: a retrospective observational study of patients in the prehospital setting. Eur J Emerg Med. 2019;26(6):433–9.",
"volume": "26",
"year": "2019"
},
{
"author": "S Alexandar",
"first-page": "7",
"issue": "83–85",
"journal-title": "Int J Pharmacol Clin Res (IJPCR)",
"key": "971_CR21",
"unstructured": "Alexandar S, Ravisankar M, Kumar RS, Jakkan K. A comprehensive review on Covid-19 delta variant. Int J Pharmacol Clin Res (IJPCR). 2021;5(83–85):7.",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(21)00475-8",
"author": "KA Twohig",
"doi-asserted-by": "publisher",
"first-page": "35",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "971_CR22",
"unstructured": "Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B. 1.617. 2) compared with alpha (B. 1.1. 7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22(1):35–42.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1101/2021.09.03.21263091",
"doi-asserted-by": "crossref",
"key": "971_CR23",
"unstructured": "Khedar RS, Mittal K, Ambaliya HC, et al. Greater Covid-19 severity and mortality in hospitalized patients in second (delta variant) wave compared to the first: single centre prospective study in India. medRxiv. 2021:2021.09. 03.21263091."
},
{
"DOI": "10.1038/s41598-022-23312-8",
"author": "A Zali",
"doi-asserted-by": "publisher",
"first-page": "18918",
"issue": "1",
"journal-title": "Sci Rep",
"key": "971_CR24",
"unstructured": "Zali A, Khodadoost M, Gholamzadeh S, et al. Mortality among hospitalized COVID-19 patients during surges of SARS-CoV-2 alpha (B. 1.1. 7) and delta (B. 1. 617. 2) variants. Sci Rep. 2022;12(1):18918.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/v13040628",
"author": "AC Hurt",
"doi-asserted-by": "publisher",
"first-page": "628",
"issue": "4",
"journal-title": "Viruses",
"key": "971_CR25",
"unstructured": "Hurt AC, Wheatley AK. Neutralizing antibody therapeutics for COVID-19. Viruses. 2021;13(4):628.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-08559-5",
"author": "F Touret",
"doi-asserted-by": "publisher",
"first-page": "4683",
"issue": "1",
"journal-title": "Sci Rep",
"key": "971_CR26",
"unstructured": "Touret F, Baronti C, Bouzidi HS, de Lamballerie X. In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B. 1.1. 529 529 isolate. Sci Rep. 2022;12(1):4683.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2020.11.007",
"author": "AJ Greaney",
"doi-asserted-by": "publisher",
"first-page": "44",
"issue": "1",
"journal-title": "Cell Host Microbe",
"key": "971_CR27",
"unstructured": "Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44-57.e9.",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1101/2021.12.14.21267772",
"doi-asserted-by": "crossref",
"key": "971_CR28",
"unstructured": "Aggarwal A, Stella AO, Walker G, et al. SARS-CoV-2 Omicron: evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. medRxiv: 2021-12 (2021)"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-024-00971-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Effect of Regdanvimab on Mortality in Patients Infected with SARS-CoV-2 Delta Variants: A Propensity Score-Matched Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}